

New Early Detection Test for Pancreatic Cancer: Enzobar® Launch Announcement
Launch of Enzobar® Pancreatic Cancer Screening Test
Kousomil Inc., based in Bunkyo, Tokyo, is excited to announce the launch of its innovative pancreatic cancer screening test, Enzobar®, starting July 1, 2025. Designed for early detection of this challenging cancer, the test will be conducted via medical institutions in partnership with BML, and will take place at Kousomil's Tokyo Testing Center.
Importance of Early Detection
Pancreatic cancer remains one of the deadliest forms of cancer, with a 5-year survival rate of only 8.5%. Due to its nonspecific symptoms, many cases are diagnosed at advanced stages, making early detection critical for improving prognosis. Recognizing this urgent need, Kousomil has developed the Enzobar® test, utilizing a groundbreaking technology called “single-molecule measurement liquid biopsy” pioneered by esteemed researchers from the University of Tokyo and RIKEN Institute.
This unique method allows for the analysis of enzyme activity at the single-molecule level, enabling precise diagnostic capabilities for pancreatic cancer.
Collaborative Development and Clinical Research
The development of the Enzobar® test was achieved through collaborative research with Professor Yuuzou Kodama from Kobe University’s Graduate School of Medicine. The objective was to create a clinically applicable tool based on the advanced diagnostic techniques stemming from activity-based diagnostics. These techniques focus on evaluating enzyme functionality rather than merely measuring protein levels, promising a more profound understanding of diseases.
In recent studies, the Enzobar® test demonstrated high sensitivity and specificity in detecting pancreatic cancer. Clinical trials across 12 facilities in Japan and the USA have validated its performance, marking a significant milestone in cancer diagnostics.
Clinical Study Design
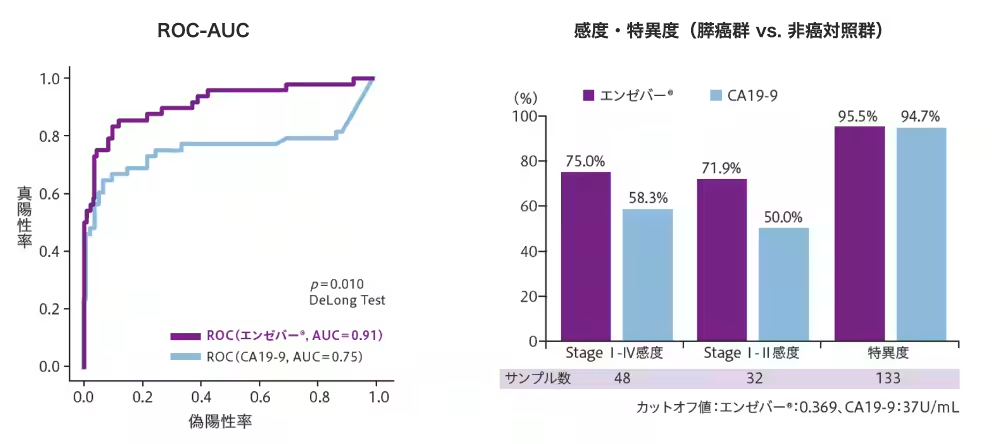
In our exploratory studies, we gathered data from 272 samples (60 pancreatic cancer and 212 non-cancer controls) for training and refined the detection criteria for further validation on an independent cohort of 181 samples (48 pancreatic cancer and 133 non-cancer controls). The results showed a sensitivity of 75.0% and a specificity of 95.5%, with an ROC-AUC of 0.91, significantly outperforming the traditional tumor marker CA19-9.
Notably, the Enzobar® test maintained a sensitivity of 71.9% for early-stage (Stage I-II) cancers, indicating its potential as an effective early screening tool. Furthermore, the positivity rate for other cancer types was only 12.7%, suggesting a high specificity for pancreatic cancer.
Future Prospects
Looking ahead, Kousomil continues its commitment to obtaining IVD regulatory approval for the Enzobar® test both in Japan and internationally. Until then, the test will be available as part of a forward-looking clinical study to further assess its efficacy and practicality in real clinical settings.
Moreover, efforts to develop screening tests for other cancer types are underway, alongside preparations to enter the United States market.
Statements from Collaborators
Toru Komatsu, Associate Professor at the University of Tokyo: “As a researcher involved in this project, I am thrilled that our pioneering enzyme activity measurement technique is being positioned to enhance early pancreatic cancer detection.”
Yuuuzou Kodama, Professor at Kobe University: “The establishment of blood-based biomarkers for early pancreatic cancer detection is a long-standing goal for specialists in this field. I am proud to see Kousomil’s innovative test enter clinical practice.”
About Kousomil Inc.
Founded in April 2022, Kousomil Inc. blends cutting-edge technologies from the research labs of Tokyo University and RIKEN. This startup emerged from the JST START program and has been recognized for its innovative contributions to early cancer diagnosis, receiving awards and grants to further its mission.
For more information and updates on the Enzobar® test, please visit Kousomil.

Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.