
iHeart Japan Secures 800 Million Yen to Advance Clinical Trials for Heart Disease Treatment
iHeart Japan Expands Its Horizons with New Funding
iHeart Japan Co., Ltd., headquartered in Kyoto and led by CEO Kenji Kakuta, has successfully secured approximately 800 million yen in funding through a third-party allocation of shares. This financing round, primarily led by Mitsui Sumitomo Insurance Capital Co., Ltd., aims to accelerate clinical trials for its innovative treatment targeting dilated cardiomyopathy (DCM), a serious form of heart disease.
Mitsui Sumitomo Insurance Capital highlighted the unique technological advantages of iHeart Japan's regenerative medical products, particularly their efficacy in treating heart failure. The core technology stems from years of research conducted by Professor Yamashita at the University of Tokyo, indicating the potential for this treatment to be applicable to multiple diseases and organs in the future.
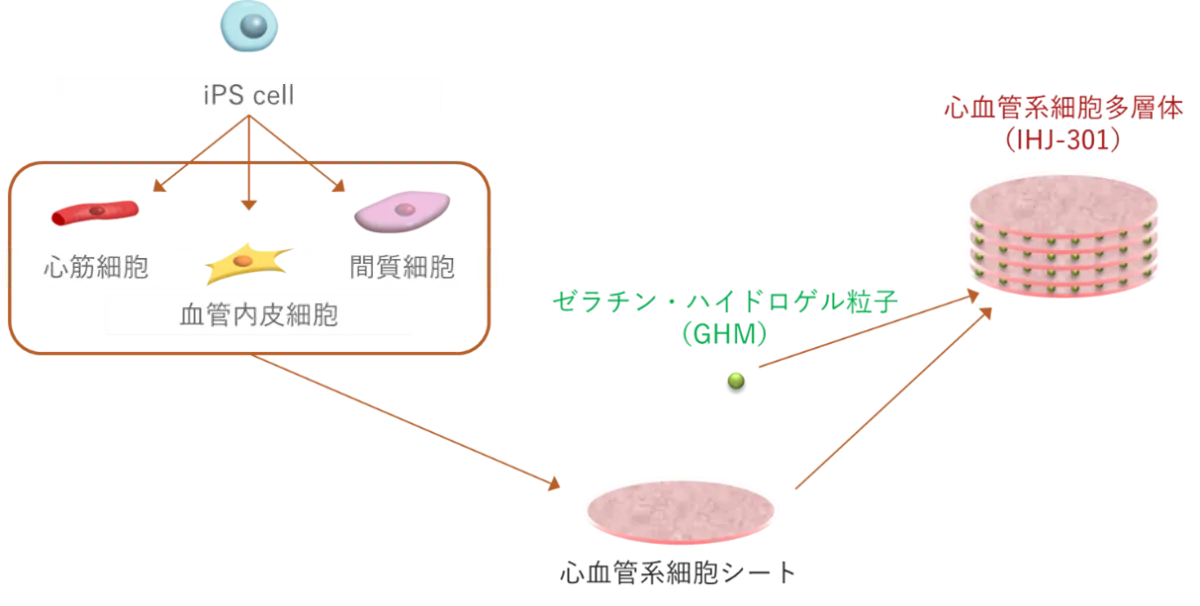
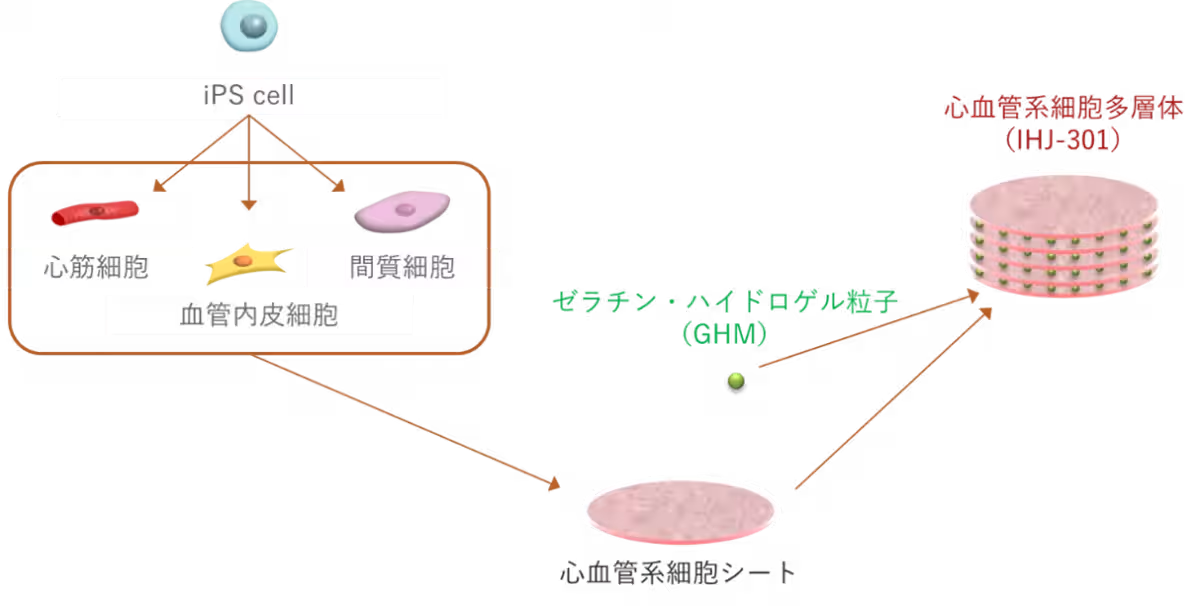
The funding will support iHeart Japan's clinical trials utilizing a product derived from human iPS cells known as IHJ-301, which aims to revolutionize the approach to heart failure. The company envisions making regenerative medicine a commonplace treatment rather than a specialized one, ultimately improving the quality of life for those affected by this condition.
Understanding Dilated Cardiomyopathy (DCM)
Dilated cardiomyopathy (DCM) is a rare disease that results in the enlargement of the heart’s chambers and thinning of the heart muscle, affecting approximately 20,000 people in Japan. It is often linked to genetic mutations or viral infections, although many cases remain unexplained. Traditional treatments focus on managing symptoms, with heart transplants being the only potential cure — a process fraught with challenges such as donor shortages and long waiting times.
iHeart Japan's IHJ-301 is a hybrid product composed of cardiovascular cells derived from iPS cells and gelatin hydrogel particles, enhancing cell survivability and therapeutic effectiveness, as evidenced by animal studies.
The clinical trial for IHJ-301 plans to evaluate a total of 10 patients, beginning with three participants for safety assessments. The inclusion criteria specify patients classified as NYHA Class II or III heart failure, with a left ventricular ejection fraction (LVEF) between 15% to 40%.
The Path Forward
Since its establishment in 2013, iHeart Japan has dedicated itself to the quest of making heart transplants unnecessary through innovative regenerative therapies. With robust patent protections for its technologies in major markets, the company is well-positioned to lead advancements in the field of regenerative medicine. In 2021, iHeart Japan was selected as a part of the Ministry of Economy, Trade and Industry's J-Startup program, reflecting its potential for high-growth impact on the healthcare landscape.
As the clinical trials progress, iHeart Japan aims not only to make significant strides in treating dilated cardiomyopathy but also to inspire hope in patients with limited options. The future of heart disease treatment looks promising as iHeart Japan paves the way for revolutionary therapies in regenerative medicine.
For more information, visit iHeart Japan Company.
Contact Information
For inquiries, please reach out to the Public Relations department at iHeart Japan via email: [email protected].
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.