
Cardiovalve Submits CE Application Following TARGET Study Success
Cardiovalve Submits CE Application Following TARGET Study Success
In a significant advancement for cardiac treatment, Venus Medtech (Hangzhou) Inc. has announced that their subsidiary, Cardiovalve, has completed the necessary documentation for a CE approval application for their innovative transcatheter tricuspid valve replacement system (TTVR). This milestone follows the promising results of the TARGET study, which evaluated the safety and efficacy of the Cardiovalve system in 150 patients across multiple European sites, including the UK and Canada.
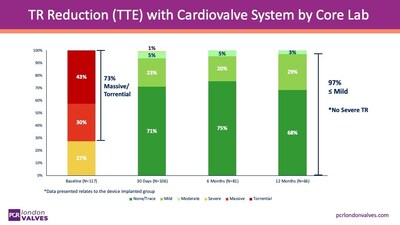
The preliminary results of the TARGET study were shared during the PCR London Valves 2025 conference by Prof. Georg Nickenig, who highlighted the system's effectiveness in addressing tricuspid regurgitation (TR), a common heart condition. The study demonstrated that the TTVR procedure successfully mitigated severe TR symptoms in the majority of participants, with 73% initially presenting with significant TR. This statistic underlines the device's clinical efficacy and its potential to improve patient outcomes.
With the device showing an acceptable safety profile, participants' symptoms were notably improved just 30 days following treatment. Noteworthy is the ongoing observation of study participants, which will continue for up to five years. Collectively, data concerning important clinical outcomes, such as mortality rates and hospitalization due to heart failure, will be published shortly, contributing to a growing body of evidence supporting the Cardiovalve system.
Prof. Nickenig expressed optimism regarding the findings, stating, "The results from the Cardiovalve study are indeed encouraging, showcasing strong effectiveness in reducing TR and a favorable safety profile, particularly with the latest version of the device. This represents a major step forward, providing hope for patients suffering from TR to soon have access to an innovative and effective treatment option."
Amir Gross, CEO of Cardiovalve, emphasized the significance of the CE filing, noting, "This marks a crucial milestone, bringing Cardiovalve closer to offering a transformative treatment for patients suffering from severe mitral and tricuspid valve insufficiency. This achievement reflects the dedication of a focused team motivated by a shared vision that not only sets goals but also successfully achieves them. Our deepest thanks to the researchers, coordinators, clinical partners, and especially to the patients and their families for their trust in us."
Additionally, Lim Hou-Sen, the CEO of Venus Medtech, remarked on the promising data as a substantial leap forward for individuals affected by tricuspid insufficiency. He expressed confidence that with the submitted CE application, Cardiovalve is positioned well to attain certification and plans to launch the TR system commercially by 2027.
About Cardiovalve
Cardiovalve, a subsidiary of Venus Medtech, is a pioneer in transcatheter valve replacement technology and remains at the forefront of innovation in structural heart therapy. The company holds over 150 approved patents and boasts an experienced team along with a state-of-the-art manufacturing facility. Cardiovalve aims to provide next-generation solutions to physicians that enhance treatment outcomes and improve patient quality of life without the necessity for open-heart surgery.
About Venus Medtech
Venus Medtech (Hangzhou) Inc., a leading innovator in transcatheter heart valve solutions for structural heart diseases, has developed a comprehensive product pipeline covering all four heart valves - TAVR, TPVR, TMVR, and TTVR - along with accompanying ancillary products. The company maintains global research and development centers in China, the United States, and Israel, striving to deliver effective treatment solutions for life-threatening conditions.
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.