
First Patient Treated with Hybrid iPS Cell Therapy for Cardiomyopathy
Breakthrough in Regenerative Medicine
iHeart Japan, headquartered in Kyoto and led by CEO Kenji Tsunoda, has announced a significant milestone in regenerative medicine: the use of their innovative IHJ-301 product in the very first human patient trial. This advancement signifies a major step towards making regenerative therapies standard rather than exceptional care.
Introduction to IHJ-301
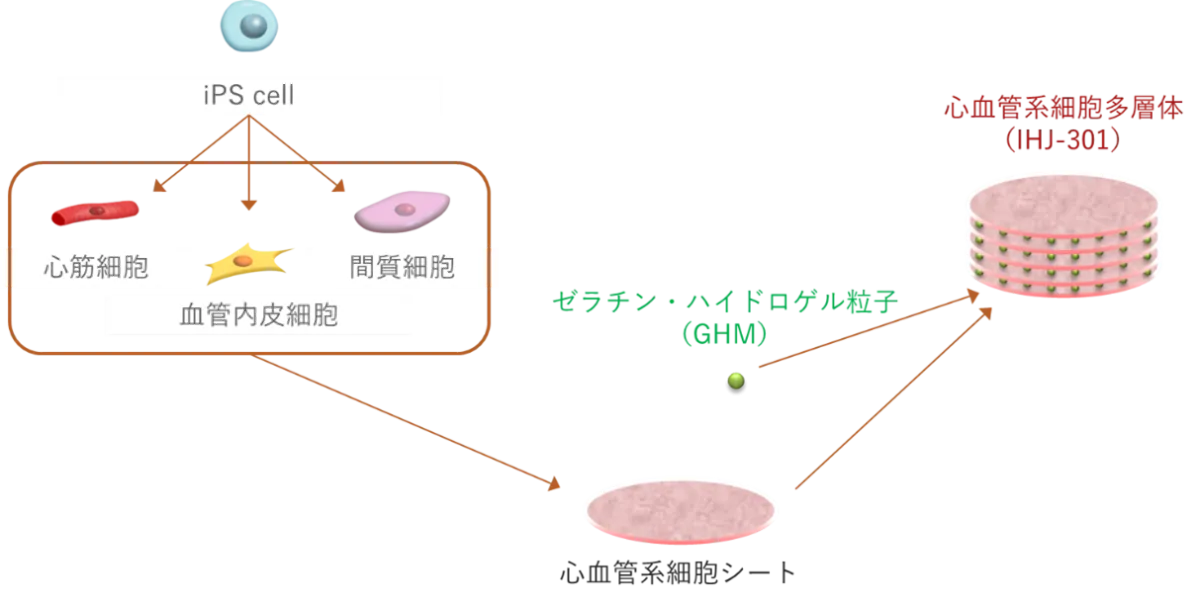
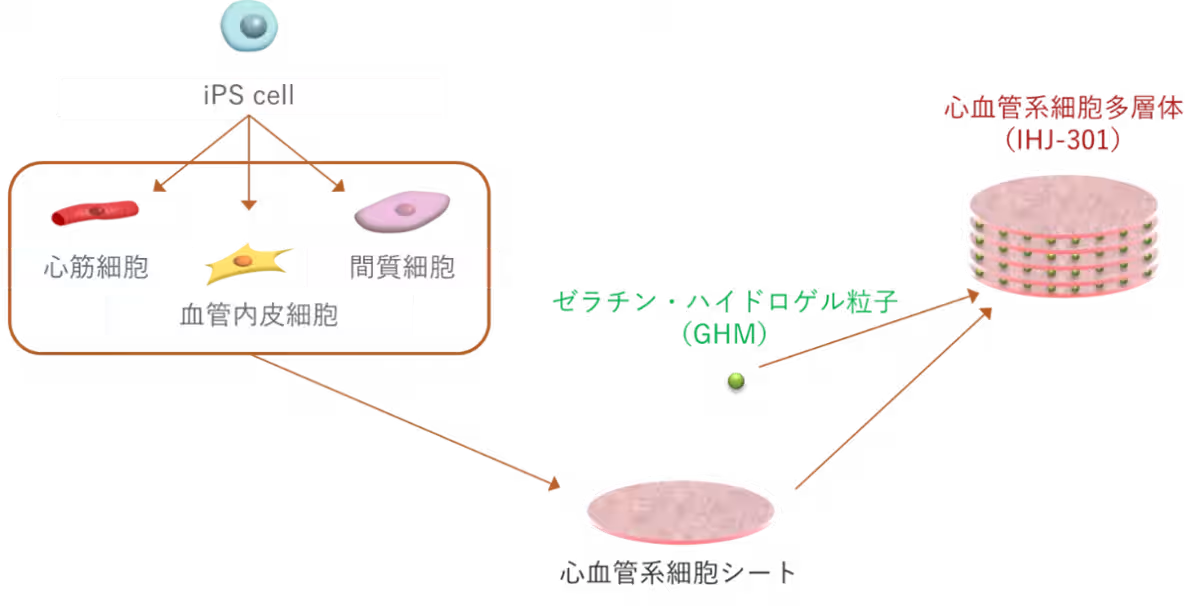
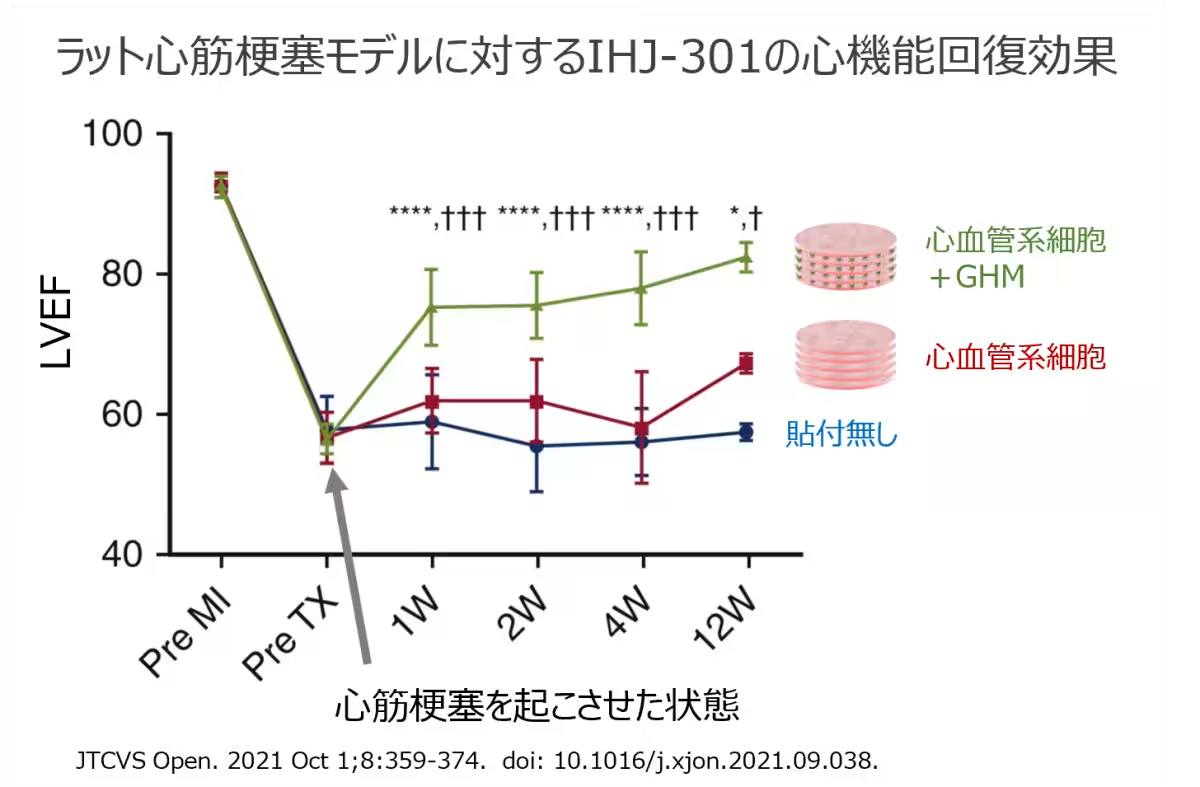
IHJ-301 is an advanced hybrid product consisting of human iPS cell-derived cardiovascular cell multilayers. Developed through cutting-edge technology that regulates cell differentiation, the product combines layers of various heart and vascular cells created from cells of healthy donors and gelatin hydrogel particles. The gelatin hydrogel enhances cell engraftment and therapeutic efficacy, as evidenced by animal studies. This novel approach was successfully used for a patient with dilated cardiomyopathy (DCM) in a clinical setting at Tokyo Women’s Medical University Hospital, on May 23, 2025.
The Patient Trial
The trial, designed to evaluate the safety and efficacy of IHJ-301, is structured in two phases with a focus on patients diagnosed with severe heart failure due to DCM. A total of ten patients are intended for inclusion, with the initial three undergoing safety assessments before proceeding with subsequent evaluations. The trial is particularly distinct because it excludes patients with pathogenic mutations in the lamin A gene. The patients receive the therapy under immunosuppressive medication to prevent rejection, with the implantation involving precise surgical techniques to apply IHJ-301 directly to the left ventricular epicardium.
Innovations in Treatment
The combination of iPS cells and hydrogel material creates a unique hybrid that not only improves the engraftment rate but also fosters cellular repair mechanisms. After six months, the immunosuppressive drugs are withdrawn, allowing the patient’s immune system to interact with IHJ-301’s constituent cells. This interaction is expected to stimulate cardiac tissue repair and potentially enhance heart function.
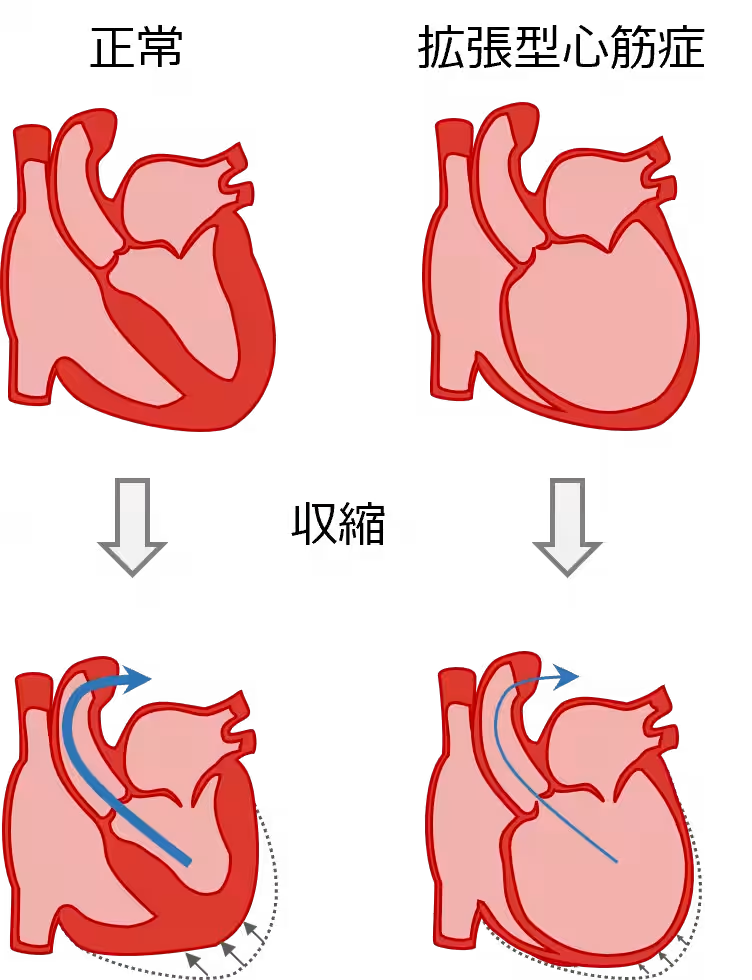
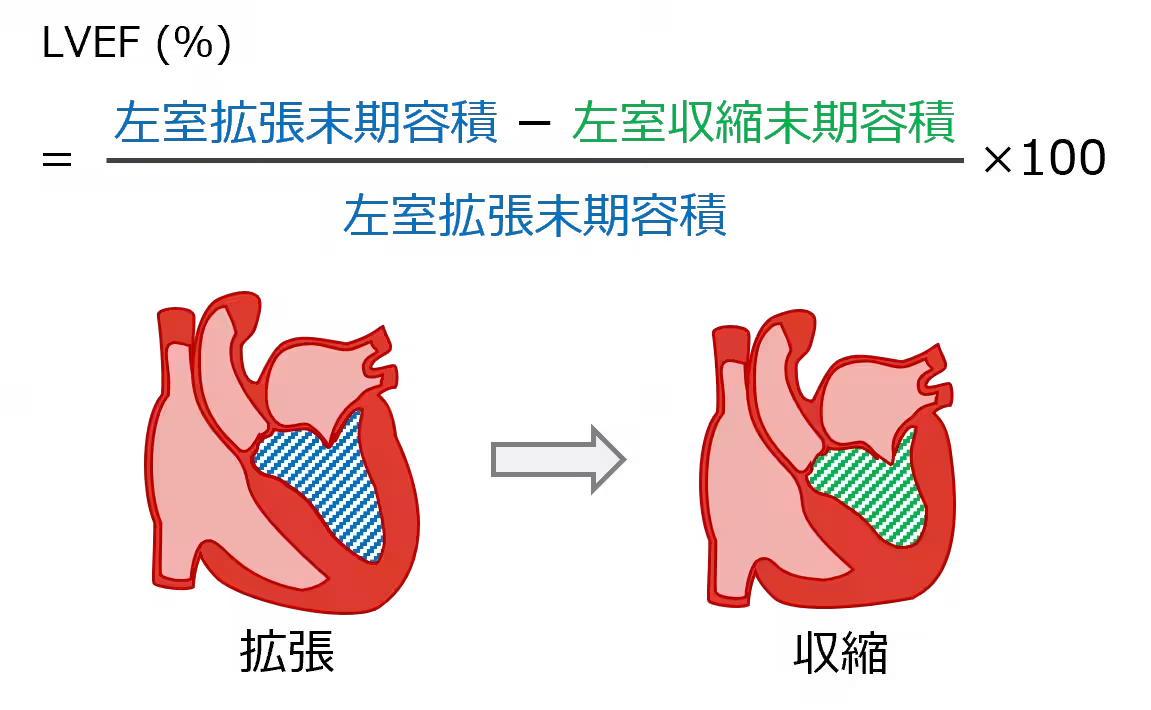
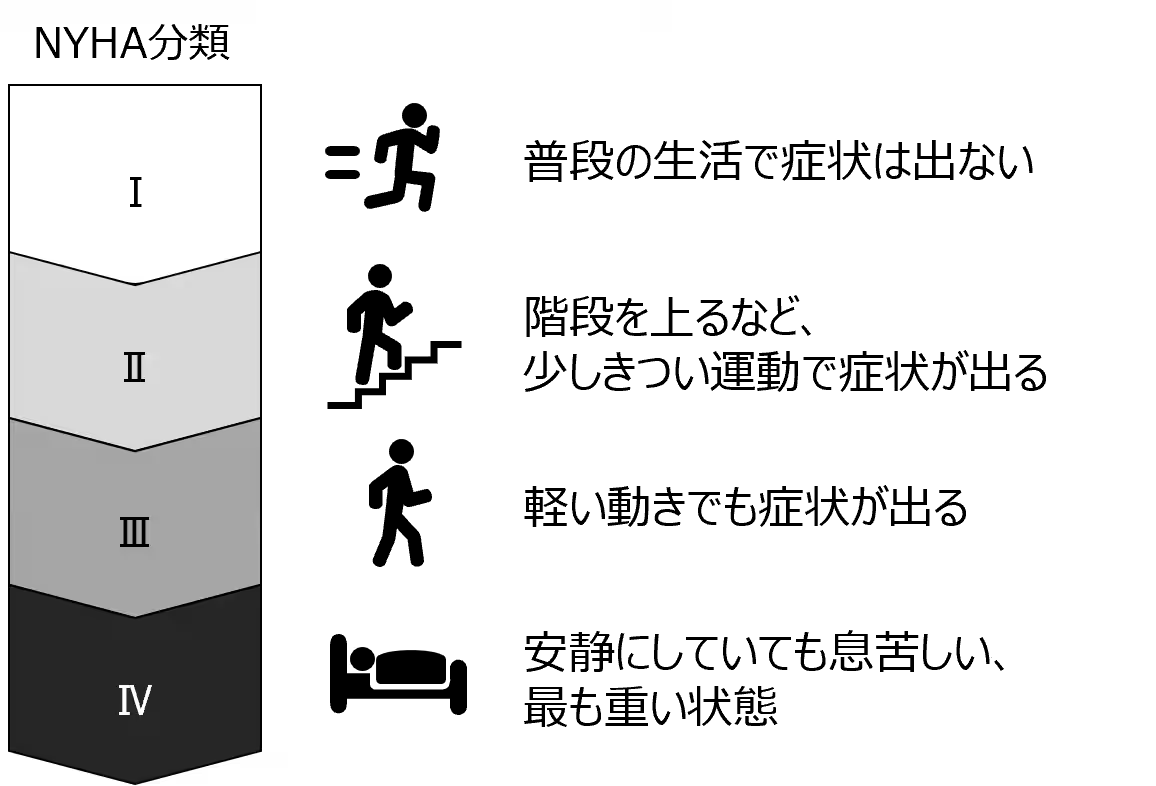
Understanding Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM) is a rare disease characterized by the dilation and thinning of the heart muscle, leading to poor cardiac output and heart failure. Currently, there are approximately 20,000 patients in Japan facing this condition, which often has no clear etiology. As heart transplantation remains the only curative treatment option, new therapies like IHJ-301 are critically needed to address donor shortages and long waiting times for transplants.
The Future of Regenerative Medicine
Since its inception in 2013, iHeart Japan has championed the vision of creating a society where heart transplants are unnecessary. The research team, including esteemed advisors such as Jun Yamashita, a professor at the University of Tokyo, has been recognized for their contributions to the field. Their innovative efforts led to the receipt of the Japan Academy Prize for collaborative research in 2016 and their selection as part of the 'J-Startup' initiative by Japan's Ministry of Economy, Trade and Industry in 2021.
As iHeart Japan progresses with this landmark study, the hope is not only to improve the lives of those suffering from DCM but also to pave the way for broader applications of regenerative therapies in treating various conditions. The company's commitment to transforming health outcomes through advanced medical research stands as a promising beacon for the future of medical practice in Japan and beyond.
For more information, please contact: [email protected]
About iHeart Japan: Learn more about their initiatives at iHeart Japan.

Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.