
Successful Development of Efficient Synthesis Method for PROTACs Through Consecutive Click Reactions
Efficient Synthesis of PROTACs Through Consecutive Click Reactions
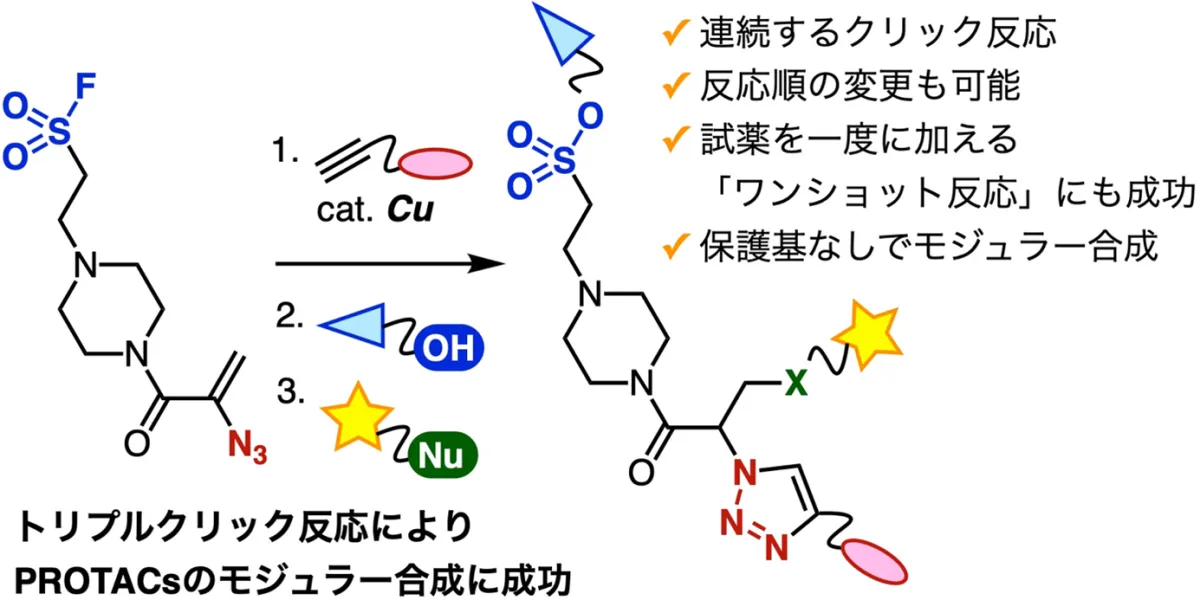
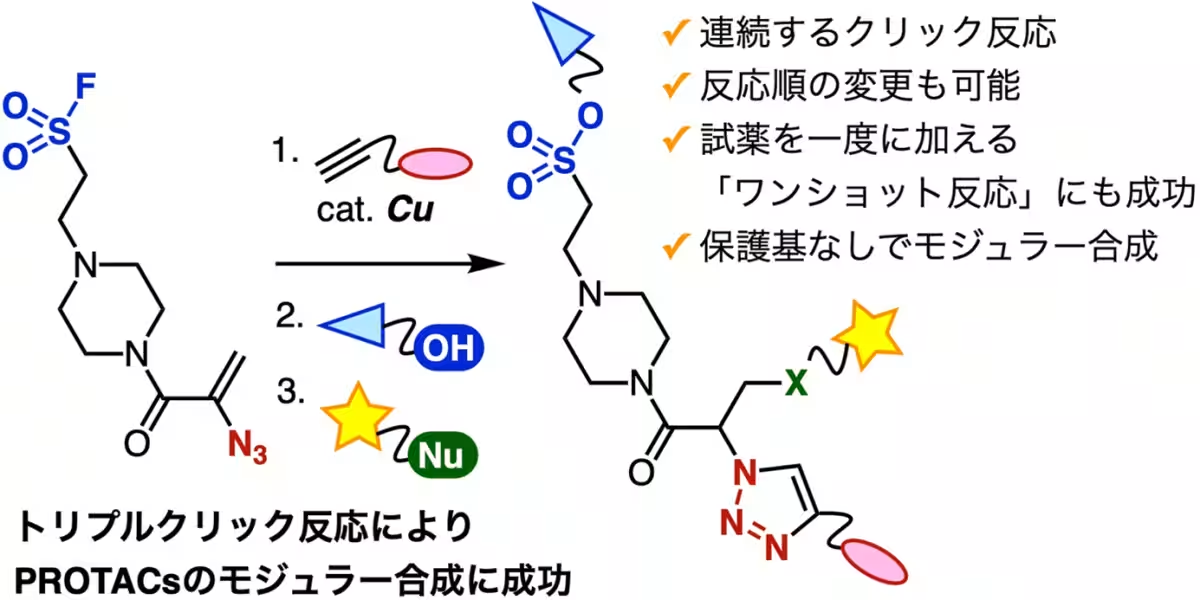
Recent advancements in the field of drug development have led to a significant breakthrough by researchers at Tokyo University. They successfully developed a novel method for synthesizing PROTACs (Proteolysis Targeting Chimeras) using consecutive click reactions. This innovative approach enables rapid assembly of three distinct functional molecules without the need for protective groups, promising a faster route for drug discovery and development.
The team, led by Associate Professor Suguru Yoshida from the Department of Life Science Engineering, comprised talented graduate students and researchers including Yuri Taninaga, Gaku Orimoto, Kaho Yamada, and Maho Miyamoto, alongside experts from the National Institute of Health Sciences, such as Hidetomo Yokoo and Yosuke Demizu. Together, they created a trifuntional platform molecule that allows for the simultaneous connection of diverse compounds through a series of efficient chemical reactions.
The Mechanism of Consecutive Click Reactions
Click chemistry is a method used for quickly and reliably synthesizing new compounds by joining molecules through specific chemical reactions. The concept gained global recognition, leading to the Nobel Prize in Chemistry awarded to Carolyn Bertozzi, Morten Meldal, and K. Barry Sharpless in 2022 for their pioneering work. This method stands out for its ability to create complex compounds without the need for multi-step processes, significantly increasing yield rates.
In this research, the team successfully employed consecutive click reactions, enabling them to rapidly synthesize PROTACs. Specifically, they initiated the process with the CuAAC (Copper-catalyzed Azide-Alkyne Cycloaddition) reaction between the alkyne derivative linked to the VHL E3 ubiquitin ligase ligand VH032 and the azide group in the platform molecule. This initially led to the creation of a triazole intermediate, achieving a remarkable yield of 94%.
Next, they performed a selective SuFEx (Sulfur-Fluorine Exchange) reaction that linked the fluorosulfanyl group from the intermediate with the hydroxyl group of an EGFR ligand, culminating in the successful synthesis of a PROTAC. Notably, this series of reactions preserved multiple functional groups without requiring protective measures. After the completion of these stages, the remaining acrylamide site was utilized to bind a fluorescent thiol via a Michael addition, thus creating a fluorescently labeled PROTAC.
Implications and Future Developments
This pioneering modular approach to PROTAC synthesis facilitates the easy incorporation of ligand sites and functional units into the molecule. As a result, it opens the door for accelerated exploration and development of new drugs, leveraging the ease of modifying chemical properties and structures.
Professor Yoshida highlighted the significance of this research, stating, "The synthesis of molecules with multiple functional groups had historically been constrained by various challenges, including the need for protecting and deprotecting groups. Our dedicated efforts in exploring modular click chemistry methods have brought promising advancements in this area. We expect that these techniques will revolutionize the efficient development of pharmaceuticals and other chemical products."
The research outcomes were published online in the esteemed journal, the Bulletin of the Chemical Society of Japan, and are set to contribute to future innovations in drug discovery and material chemistry.
Conclusion
This remarkable research underscores the potential of click chemistry and modular synthesis methods in revolutionizing the pharmaceutical landscape. By simplifying the PROTAC synthesis process, researchers are poised to make significant strides in drug development, ultimately benefiting public health and therapeutic advancements.
The study was conducted with support from the Japan Society for the Promotion of Science (Grants 23K17920, 23H04926) and the Asahi Glass Foundation's funding.
For more insights, you can access the detailed study in the Bulletin of the Chemical Society of Japan, DOI: 10.1093/bulcsj/uoaf115.
Topics Health)








【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.