
Innovative Method for Generating Human Platelet Lysate from Waste Blood Products
Innovative Method for Generating Human Platelet Lysate from Waste Blood Products
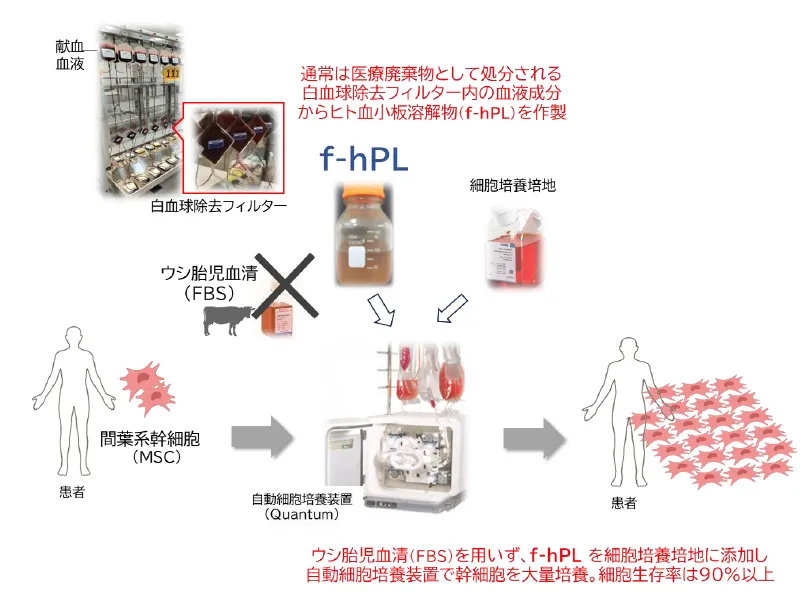
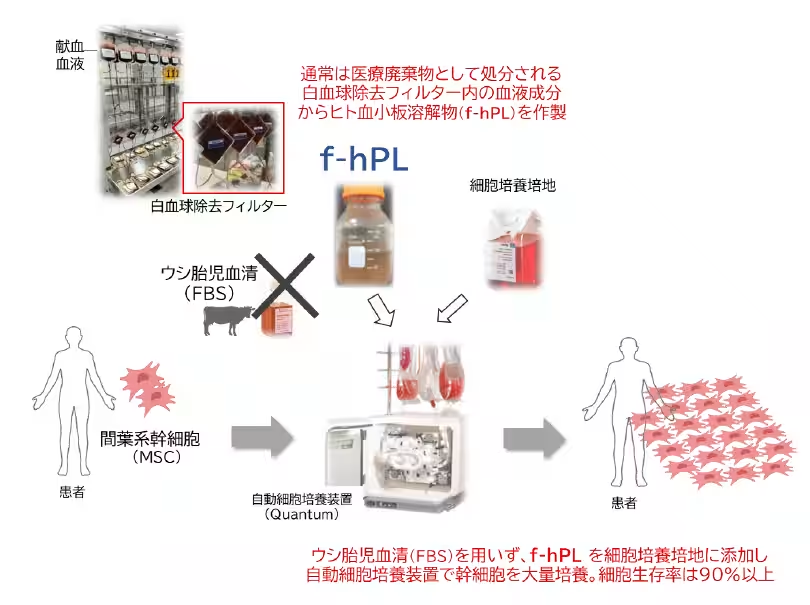
Researchers at Hokkaido University, in collaboration with RAINBOW Inc. and the Hokkaido Block Blood Center of the Japanese Red Cross, have successfully developed a new method for producing human platelet lysate (f-hPL). This innovative f-hPL is derived from discarded leukoreduction filters, which typically contain residual platelets and plasma components. This breakthrough not only enhances the quality of regenerative medicine products but also addresses sustainability concerns in medical waste.
Background
The practical application of regenerative medicine and cell therapies requires the mass proliferation of cells. Traditionally, fetal bovine serum (FBS) has been the standard supplement used in cell culture. However, FBS presents several challenges, including immunological reactions, ethical concerns, and the risk of zoonotic infections. f-hPL has emerged as a promising alternative, yet securing sufficient human-derived materials for clinical use has been challenging. By focusing on the discarded leukoreduction filters used in blood product manufacturing, the research team identified a viable source for high-quality f-hPL.
Research Methodology
In their study, the researchers concentrated on leukoreduction filters that are part of the blood product manufacturing process. They developed a method for efficiently recovering and processing the residual platelets and plasma from these filters, establishing a safe and effective production process for f-hPL.
Through this technique, approximately 3.5 × 10^10 platelets can be retrieved from a single filter, achieving an average recovery rate of 37.1%. The produced f-hPL, formulated at an optimal protein concentration of 27 mg/mL, demonstrated MSC proliferation capabilities that are four times greater than standard FBS and comparable to commercially available hPL. The mesenchymal stem cells (MSCs) derived from this process met the International Society for Cellular Therapy (ISCT) standards, exhibiting the necessary surface markers and maintaining differentiation potential into three lineages: adipose, bone, and cartilage.
Moreover, the research team successfully scaled up production using the Quantum automated cell culture system, achieving over 90% cell viability. This technique is essential for moving forward with clinical applications.
Significance of Findings
The implications of this research are profound.
1. Sustainable Resource for Regenerative Medicine
Utilizing by-products from blood product manufacturing offers a low-cost and sustainable foundation for cell therapies. This addresses the critical challenge of stable platelet supply in hPL products.
2. Accelerated Development of Regenerative Medicine Products
The newly developed f-hPL shows significantly superior MSC proliferation compared to FBS and commercial hPL, with the potential for high-quality clinical-grade cell products. The cell aging suppression and retention of differentiation capabilities further enhance its applicability in GMP manufacturing processes.
3. Recycling Medical Waste and Contributing to SDGs
Previously treated as medical waste, leukoreduction filters can now be viewed as renewable resources, contributing to a resource circulation model in healthcare. This aligns with the United Nations Sustainable Development Goals, particularly Goal 12, focusing on responsible consumption and production.
Future Expectations
Moving forward, the research team aims to enhance collaboration with academic institutions and companies to develop GMP-compliant manufacturing processes and explore various clinical research applications. They also envision commercializing f-hPL products and establishing an international supply chain, making significant contributions to the popularization and commercialization of regenerative medicine.
Acknowledgments
This research has been supported by the Ministry of Economy, Trade and Industry Go-Tech program, RAINBOW Inc., and the Japan Agency for Medical Research and Development.
Publication Information
The research article titled Human platelet lysate produced from leukoreduction filter contents enables sufficient MSC growth will be published online on April 23, 2025, in the journal Stem Cell Research & Therapy by Springer Nature. This highlights the global importance of the findings in the stem cell and regenerative medicine fields.
For further inquiries, interested parties can contact:
- - Masahito Kawabori, Hokkaido University, email: [email protected]
- - RAINBOW Inc., email: [email protected]
- - Akimitsu Akinoumi, Hokkaido Block Blood Center, email: [email protected]
- - Sumio Ohtsuki, Kumamoto University, email: [email protected]
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.