

Accelerating Drug Discovery with One-Step Benzene Ring Transformation
Introduction
In a groundbreaking development, researchers from Waseda University have unveiled a novel reaction that enables the one-step conversion of benzene rings into various heteroaromatic compounds. This innovative method, which utilizes classic Claisen and reverse Claisen reactions, significantly speeds up the drug discovery process by simplifying complex reactions that previously required multiple steps.
Significance of Heteroaromatic Swapping
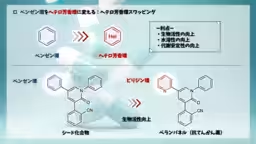
Heteroaromatic swapping is a critical technique in pharmaceutical development, enhancing the solubility and stability of drugs. Traditional methods required a lengthy multi-step process, making it less efficient and versatile. The newly developed reaction not only replaces benzene rings with nitrogen or oxygen-containing heteroaromatic rings in a single step but does so with high efficiency, making it applicable to complex drug molecules and natural products.
Achievements of the Research Team
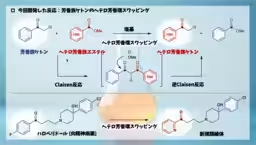
The study, led by Professor Junichiro Yamaguchi and his team, demonstrates that aromatic ketones can be mixed with heteroaromatic esters, achieving the creation of a wide variety of heteroaromatic ketones efficiently. The researchers found that the reaction proceeds smoothly under mild conditions, showcasing its potential in drug synthesis. For instance, the method allows for the conversion of haloperidol, an antipsychotic drug, into new derivatives with heteroaromatic rings, including over 25 variations.
Implications for Drug Development
The practical implications of this technique are profound. By achieving structural modifications in one step, researchers can rapidly synthesize numerous candidate compounds, thus accelerating the search and optimization of new drugs. This development also challenges existing beliefs about classical Claisen reactions, showing that intermediate stabilities can allow reverse Claisen reactions to proceed, potentially reshaping academic teachings in organic chemistry.
Challenges Ahead
Despite its broad applicability, this new method does face limitations. Not all substrates can yield the desired reaction, especially electronically rich heteroaromatic compounds or certain simple ketones, indicating a need for expansion in substrate applicability. Furthermore, the requirement for a ketone adjacent to the benzene ring poses constraints, which the research team aims to address through further exploration and innovation.
Future Research Directions
To enhance the versatility of this method, the team is working on developing new modifications that can accommodate a wider range of benzene-based compounds. The potential applications of this technique in drug candidates and functionalized compounds could lead to significant advancements in drug discovery and material development.
Conclusion
The revelation of exceptions to long-held chemical rules offers a moment of excitement and achievement for the research team. They believe that this straightforward and practical method will be a valuable asset for fellow researchers in drug discovery. Moving forward, they intend to continue refining organic chemistry's foundational reactions to contribute to society through advanced molecular transformation techniques.
Acknowledgments
This research was supported by the Japan Society for the Promotion of Science (JSPS) through multiple funding programs. The team looks forward to exploring more in this promising area of chemical research.


Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.