
The Role of Protein Aggregation in Neural Cell Differentiation Revealed by ATRX Phase Separation
Uncovering the Mechanism of Neural Differentiation Through ATRX's Liquid-Liquid Phase Separation
Recent discoveries in neuroscience continue to reveal the intricate mechanisms that govern brain development and neural differentiation. A collaborative research team from Waseda University, Tokyo Medical University, and Keio University has uncovered a significant finding regarding the role of the protein ATRX. Known for its involvement in X-linked intellectual disability and developmental disorders, ATRX operates through a unique process called liquid-liquid phase separation (LLPS), forming condensates that appear to play a critical role in the differentiation of neural cells.
The research, led by Ryo Tomooka, a research assistant at Waseda University, alongside Jun Komiyama, a professor at Tokyo Medical University, and Hideyuki Okano, a center director at Keio University, recently published their findings in Nature Communications on July 14, 2025. The study highlights that ATRX facilitates neural differentiation by regulating the expression of genes essential for this process through its ability to form cohesive liquid-like droplets within the nucleus of cells.
Key Findings and Methodology
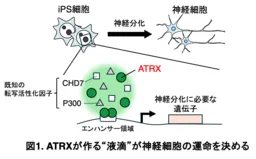
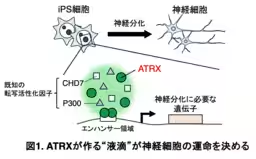
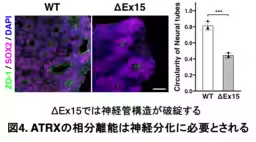
Through their investigation, the team discovered that when ATRX forms these intracellular condensates, it effectively creates a microenvironment conducive to gene expression crucial for neural differentiation. The absence of ATRX or disruption of its condensation abilities can lead to defective differentiation pathways, resulting in abnormal neural tube structures and potentially severe brain developmental issues.
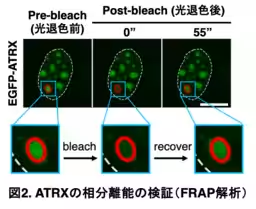
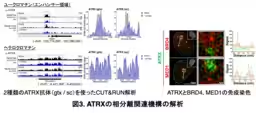
Utilizing human induced pluripotent stem cells (iPSCs), the researchers focused on the intrinsically disordered region (IDR) of ATRX, known for facilitating LLPS that influences gene expression dynamics. Advanced techniques, including fluorescence recovery after photobleaching (FRAP), allowed them to observe the fluid nature of these condensates and how they interact with various chromatin states within the nucleus. Their findings elucidated the relationship between ATRX-induced phase separation and the operational centers of active gene expression, particularly within active euchromatin regions.
Furthermore, by employing CRISPR/Cas9 genome editing, the researchers created mutations that affected the condensate formation capability of ATRX. Their experiments demonstrated that the poly-E domain, rich in glutamine residues, was essential for the formation of these aggregates. Without this domain, neural differentiation was significantly impaired, affirming ATRX's critical role in the developmental process.
Implications for Understanding Disorders
The implications of this research extend far beyond the laboratory. The discovery that ATRX contributes to the regulation of gene expression through LLPS presents a paradigm shift in understanding how genetic mutations, such as those resulting in ATR-X syndrome or certain types of glioblastoma, can lead to developmental disorders and malignancies. With ATRX's emerging role in both transcriptional activation and chromatin remodeling, the potential for developing targeted therapies that aim to restore or mimic its function is vast. This new pathway might aid in the development of innovative treatment strategies for various neurodevelopmental disorders associated with ATRX mutations.
Future Research Directions
The research team emphasizes the necessity for continued exploration into the mechanisms governing ATRX aggregation and its broader impacts on other neurodevelopmental diseases. Developing therapeutics aimed at enhancing or modulating ATRX's condensate formation presents an exciting avenue for clinical applications. Moreover, understanding the controlling mechanisms behind this aggregation can help decipher its role in different contexts of brain pathology.
In conclusion, the findings reveal a stunning complexity in how proteins like ATRX act as architects of neural cell fate through their capacity for phase separation. This transformative insight reinforces the need for innovative research methodologies to further unravel the underlying molecular dynamics that govern neural development and offers a beacon of hope for understanding and treating related disorders. Future studies will inevitably shed light on the intricate interplay between genetic factors and their environmental influences, propelling forward our understanding of developmental neurobiology.
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.