
New Insights on Lactate Oxidase Functionality with Sucrose Monolaurate Under Acidic Conditions
Advancements in Lactate Oxidase Functionality: The Role of Sucrose Monolaurate
Recent research from the Faculty of Applied Chemistry at Tokyo University of Science has made significant strides in enhancing the functionality of lactate oxidase (LOx) in acidic environments by utilizing sucrose monolaurate. This study, spearheaded by Associate Professor Isao Shitanda and his team, shows that LOx can maintain approximately 80% of its activity at pH 5.0, compared to 50% without this stabilizing agent. This advancement could have crucial implications for the development of wearable devices capable of monitoring metabolites in human sweat.
Background of the Study
With the rise in demand for wearable technology, especially devices that monitor metabolic changes through bodily fluids like sweat, the stability of enzymes such as LOx becomes paramount. Notably, sweat can become acidic, compromising the functionality of these enzymes. Researchers have identified sucrose monolaurate, a type of sugar-based surfactant, as an effective stabilizer that protects LOx from inactivation due to these acidic conditions.
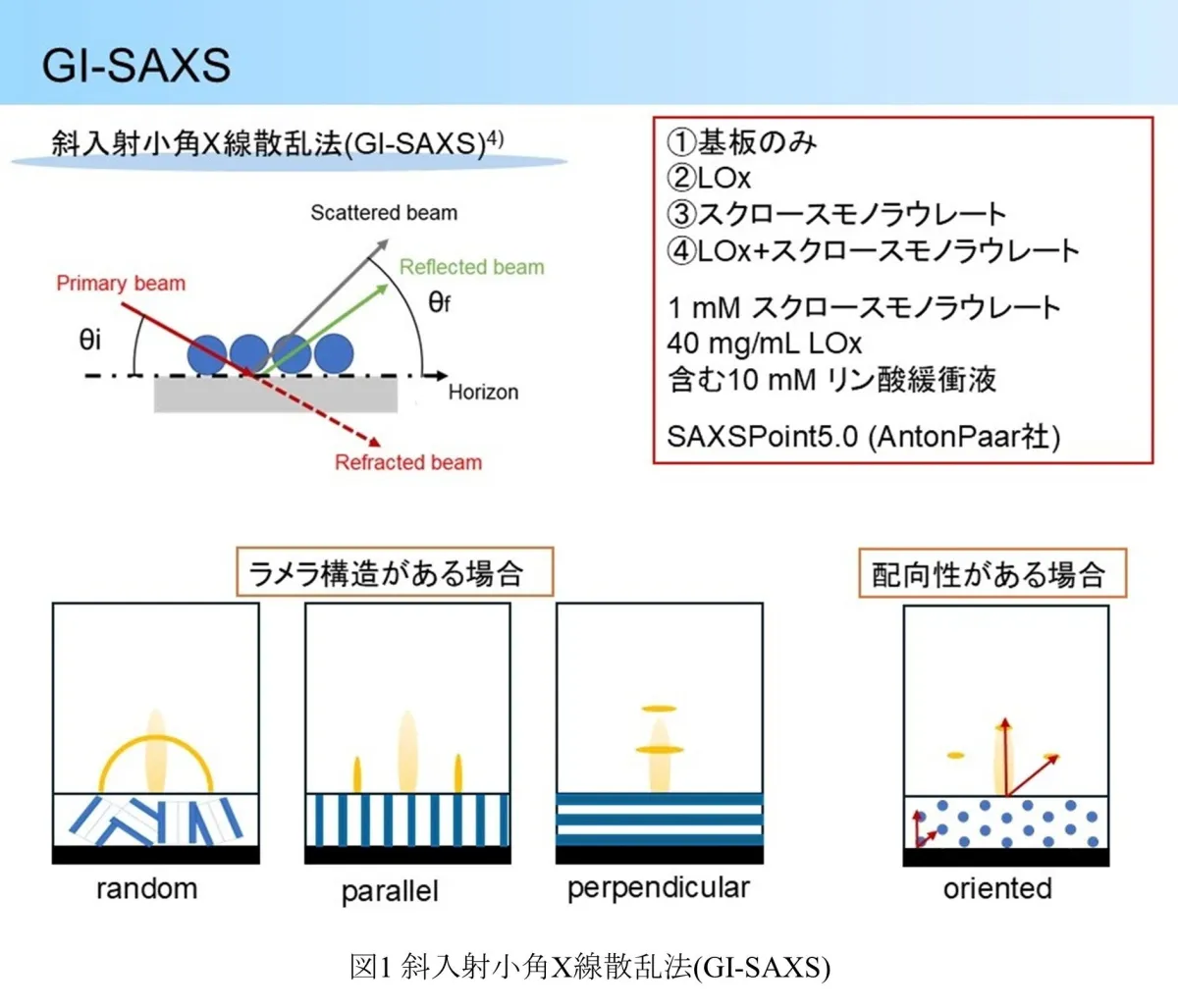
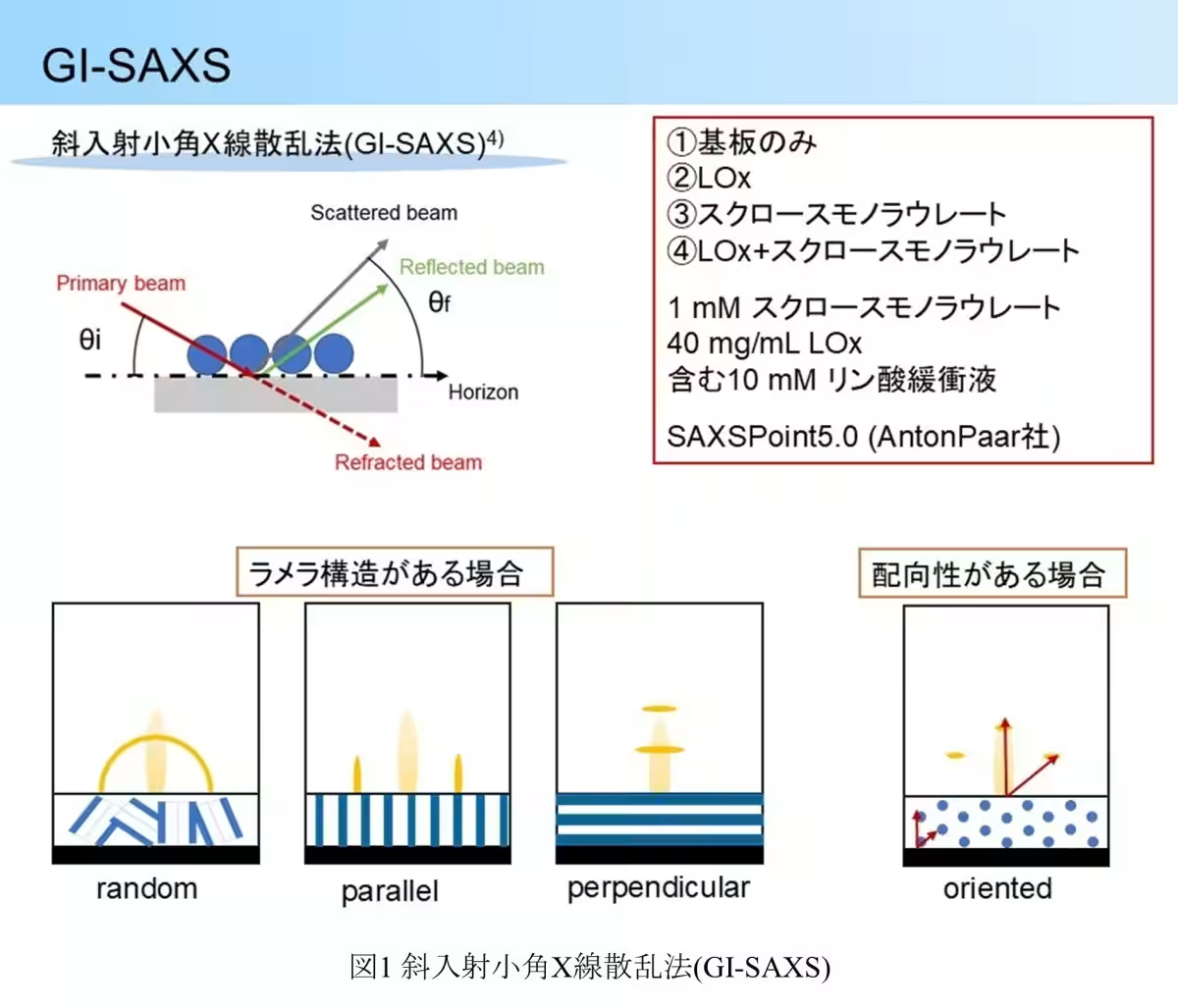
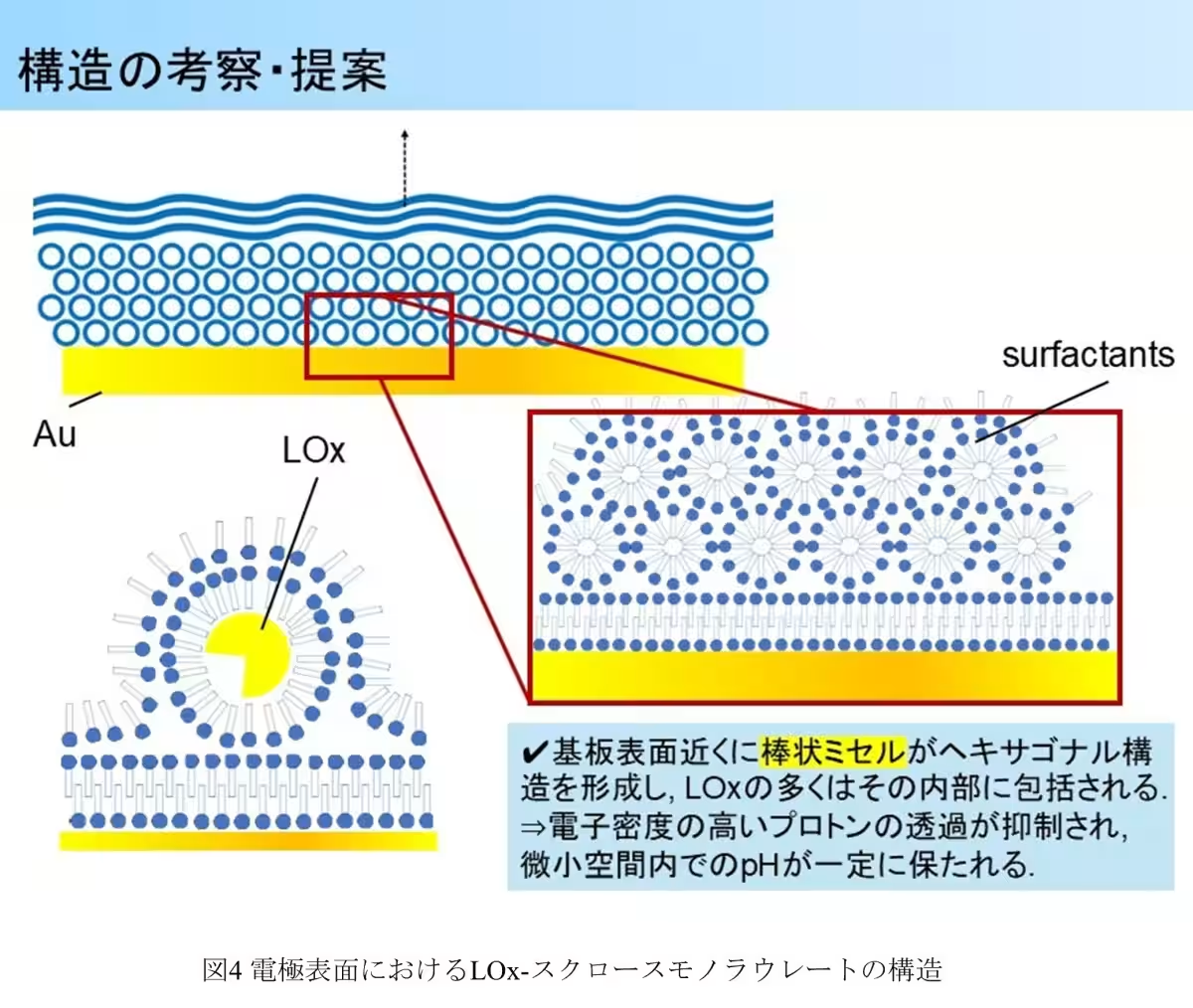
Utilizing grazing incidence small-angle X-ray scattering (GI-SAXS), the researchers unveiled detailed structural arrangements at the enzyme electrode surface, illustrating the unique configurations formed by sucrose monolaurate that encapsulate LOx, ensuring its stability.
Key Outcomes of the Research
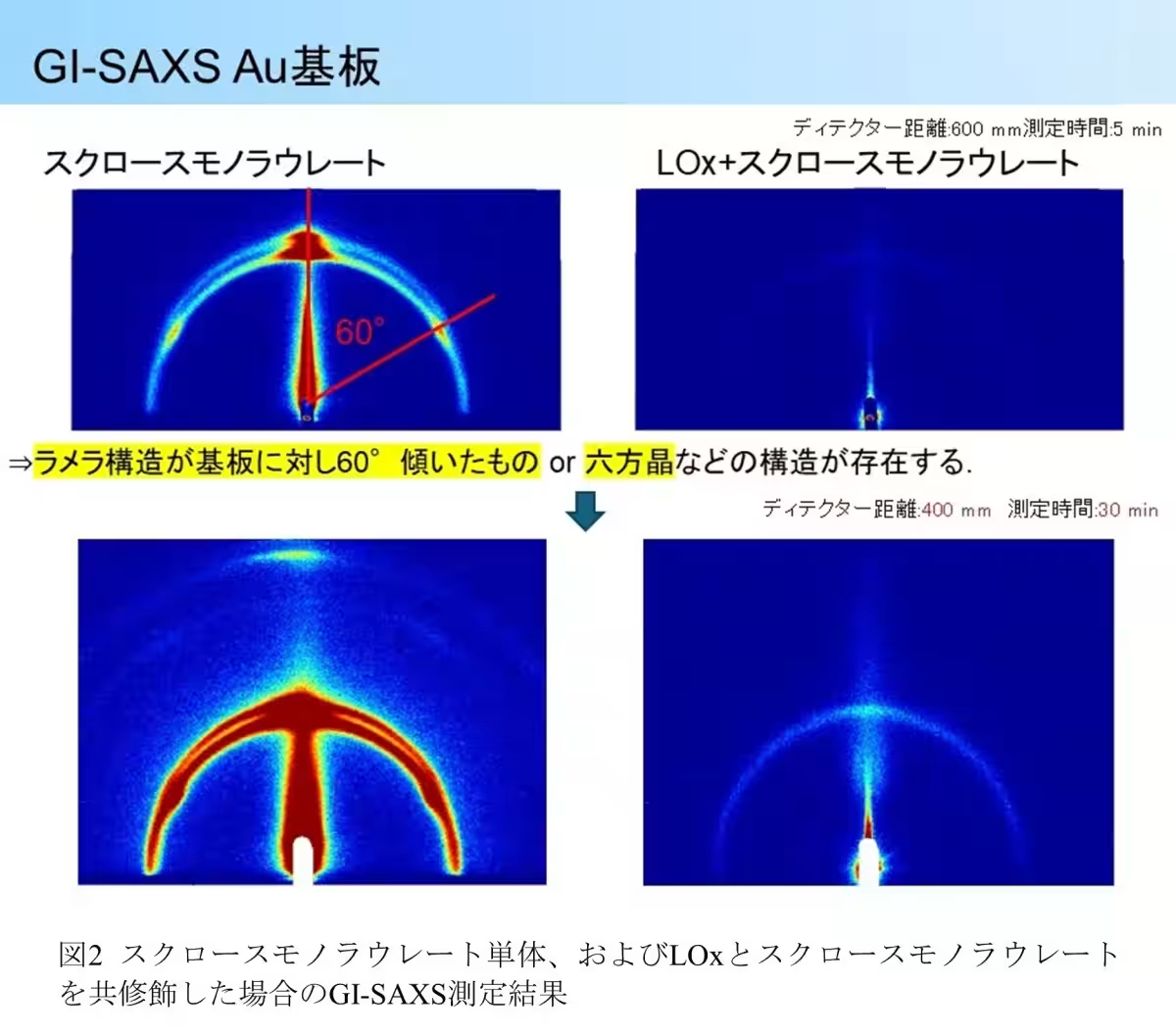
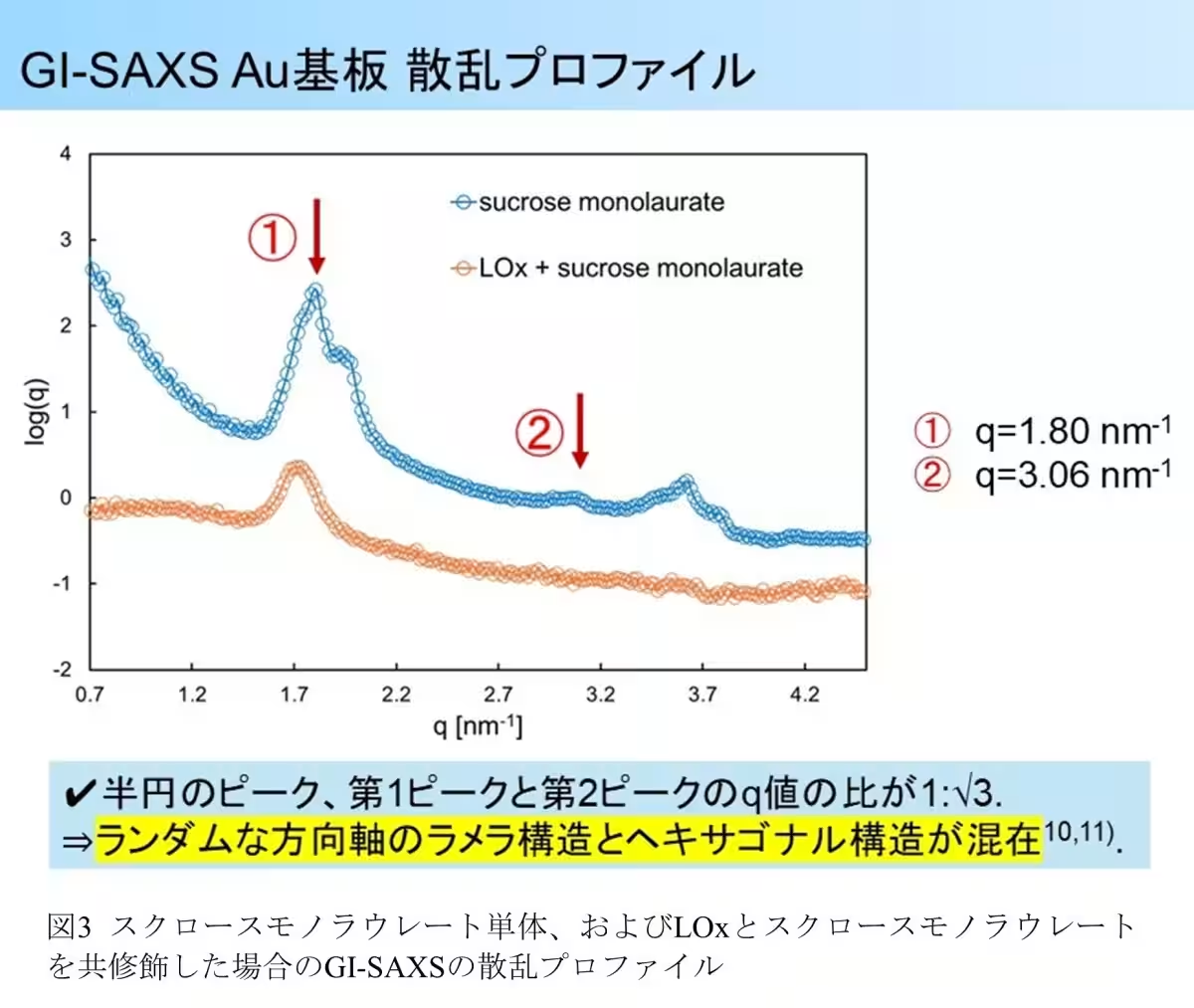
One of the most significant findings was the structural formation of a hexagonal arrangement accompanied by a layered structure induced by sucrose monolaurate on the electrode surface. The core-shell micelle structure formed by sucrose monolaurate provided a protective mechanism that secures LOx from fluctuations in pH while allowing the necessary substrates to access the enzyme.
At neutral pH levels, LOx displayed optimal activity, but as the pH dropped to 5.0, the active rate without any stabilizing agent plummeted to 50%. In contrast, the LOx electrodes modified with sucrose monolaurate retained 80% activity, highlighting its efficacy as a stabilizer in various acidic conditions.
The GI-SAXS analysis further illustrated that LOx alone did not present any distinguishable structural pattern, implying a random orientation on the electrode surface. Conversely, the presence of sucrose monolaurate led to discernible hexagonal and layered structures in specific configurations, suggesting that LOx can integrate into these formations, leading to enhanced environmental stability for the enzyme.
Mechanism of Action
The success of sucrose monolaurate lies in its ability to form a structured environment around LOx. This surfactant orients itself with hydrophilic parts facing the aqueous solution, creating a protective shell that regulates the microenvironment's pH, thus maintaining the enzyme's activity even in acidic solutions. This unique protective mechanism suggests that sucrose monolaurate can selectively allow substrates to enter while restricting the passage of protons, thereby stabilizing LOx effectively.
Research leader, Associate Professor Shitanda, emphasizes the rapidly growing importance of real-time monitoring of lactate levels in sweat, which is crucial for applications in sports training management and prevention of heatstroke. This study showcases the innovative framework and methodologies employed, including GI-SAXS, to clarify how stabilizing agents like sucrose monolaurate function to enhance enzyme durability under challenging environmental conditions.
As the study is set to be published in the renowned journal 'Langmuir,' the implications of this work could lead to advanced bio-sensors and wearable technologies that promise improved health monitoring capabilities. Through ongoing research, the potential for developing high-performance bio-devices that maintain enzyme activity in acidic environments seems ever more feasible.
Conclusion
The findings highlight the necessity and effectiveness of employing stabilizing agents in enzyme applications, particularly for the burgeoning field of wearable health monitoring devices. With sucrose monolaurate emerging as a potent stabilizer, this research not only opens avenues for further exploration in enzyme technology but also reinforces the potential for breakthroughs in monitoring physiological changes in real-time.
This research was supported by the Japan Society for the Promotion of Science (JSPS) under Grant Number JP24K02819.
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.