

Next Generation Bio-Implants Begin Clinical Research in Tokyo
Next Generation Bio-Implants Begin Clinical Research in Tokyo
The innovative company OrganTech, based in Chuo City, Tokyo, is leading the charge in the application of groundbreaking organ regeneration technology in the medical field. Their focus lies in developing next-generation bio-implants, a venture that has recently gained traction with the registration of Hillside Akasaka Dental Clinic as a designated clinical research institution. Starting in August 2025, this clinic - located in Minato City - will start conducting clinical research on these advanced bio-implants.
OrganTech's pioneering research has been ongoing since 2025, with clinical evaluations conducted at the National Institute for Brain and Nerve Disorders in Koriyama City, Fukushima. This research centers on assessing the efficacy and safety of a root membrane junction type implant, particularly aimed at patients requiring tooth extraction due to various dental complications such as root fractures, crowns, caries, or dislocated teeth.
Hillside Akasaka Dental Clinic aims to provide safe, trouble-free, and long-lasting implant treatments by integrating top-quality diagnostic and treatment equipment sourced globally. They not only focus on advanced implant therapies but also address complications arising from implants placed at other facilities. Led by Dr. Usho Miyahara, a specialist with extensive training in bone regeneration research during his time in Sweden, the clinic embodies a commitment to excellence in implant treatments. Dr. Miyahara has notably passed the certification exam by the European Implant Society, further showcasing his expertise.
The objective of bio-implants is to reinvent the treatment for tooth loss through organ regeneration techniques. Unlike traditional implant therapies, which rely on bone fusion, bio-implants connect with the alveolar bone through the periodontal ligament just like natural teeth. This revolutionary approach helps retain important biological functions such as the sensory perception of chewing and infection defense, which typically diminish with conventional implants. The foundational research behind this technology is primarily attributed to Takashi Tsuji, chairman and founder of OrganTech, who spearheaded studies at RIKEN (the Institute of Physical and Chemical Research).
As a part of their mission, OrganTech is also looking to roll out bio-implants globally, contributing to the development of a high-value industry within Japan while improving quality of life and promoting healthy longevity.
Outline of the Research Study
Research Title
Evaluation of Efficacy through Attachment of Root Membrane Tissue in Tooth Extraction Sites Using Root Membrane Junction Type Implants
Target Patients
Patients requiring tooth extraction due to root fractures, crown fractures, caries, or dislocated teeth.
Inclusion Criteria
Patients must meet all the following criteria:
1. Patients requiring extraction of single-root teeth (incisors to premolars) with remaining healthy periodontal tissue around the extraction site, usually caused by caries or fractures.
2. Adjacent teeth must also be natural and possess healthy periodontal tissue, ensuring adequate occlusion with opposing teeth.
3. Consent must be obtained from patients aged 18 and above.
4. A written agreement to participate in the clinical study must be provided by the patient.
Lead Research Physician
Shohei Kasugai
Study Duration
February 1, 2025, to January 31, 2027 (estimated) - approximately 15 months from the first visit to the end of the study.
Institutions Conducting the Study
- - General Incorporated Foundation Brain and Nerve Disorders Research Institute, affiliated with Minami-Tohoku Medical Clinic
- - Hillside Akasaka Dental Clinic
Sample Size
Six cases
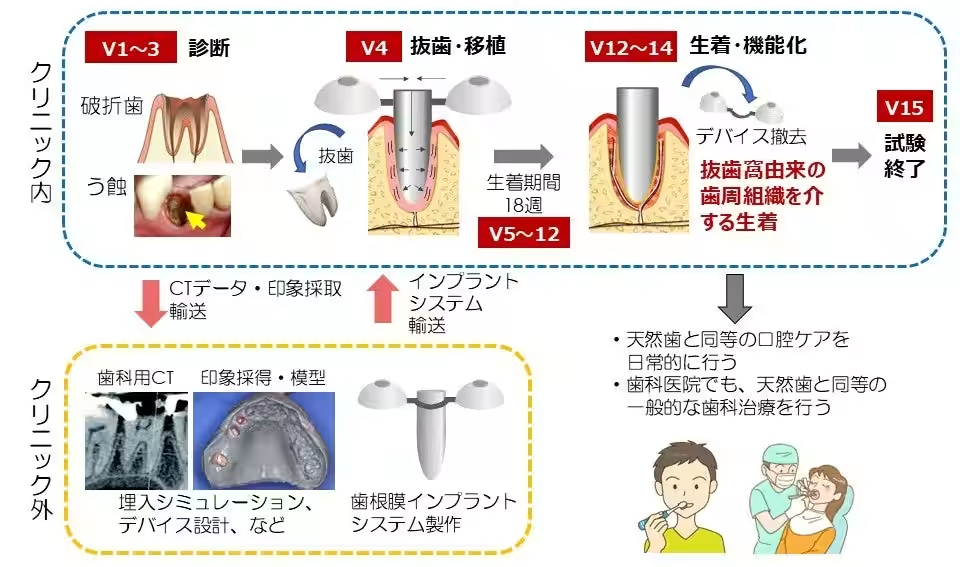
Treatment Protocol
The study involves approximately 15 patient visits over 15 months. During the first three visits, patient suitability for the treatment will be assessed, and optimal implants for the extraction site will be selected. The fourth visit involves extraction and simultaneous implantation of the selected device. Measurements will be taken over an 18-week period to ensure proper integration of the implant and formation of the periodontal ligament. Patients will be advised to refrain from hard chewing and intense brushing in the treatment area during this time. The removal of the fixation device will occur between weeks 18 and 36, concluding the treatment around one year post-procedure, allowing patients to resume normal chewing and brushing habits as they would with natural teeth.
Anticipated Risks and Management
This clinical study represents the world's first implementation of implant treatment that encourages integration through periodontal tissue connection, using unapproved devices in a ‘first in human’ trial. Although patient safety remains a priority and careful monitoring will be conducted during the procedure, potential risks such as infection at the implant site and bone absorption around the implant may arise. Should complications occur, removals or alternative treatments may be necessary. If the removal is required, standard implant treatments will be offered to ensure proper dental care.
For inquiries regarding the clinical study, please contact:
- - Contact for Hillside Akasaka Dental Clinic Research Department


Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.