
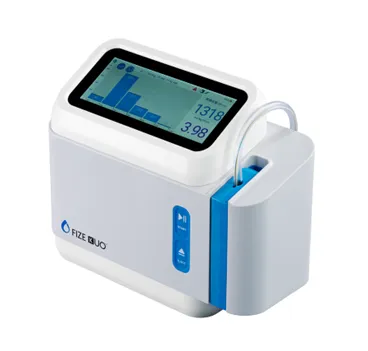
Introducing the FIZE kUO Automated Urine Monitoring System in Japan
Revolutionizing Patient Care with FIZE kUO
Asahi Kasei Medical Corporation, based in Chiyoda, Tokyo, recently announced the exclusive launch of the FIZE kUO Automated Urine Monitoring System in Japan. This innovative system, developed by FIZE Medical Ltd. from Israel, will be available for sale starting November 25.
The FIZE kUO system is designed to automatically and continuously measure urine output from both adult and pediatric patients who are equipped with bladder catheters, specifically Foley catheters. This crucial technology facilitates real-time measurement of urine volume, significantly enhancing patient monitoring, particularly in critical care settings where urine output reflects important vital signs related to kidney function and hemodynamic stability.
Enhanced Measurement Capabilities
The FIZE kUO employs built-in pressure sensors to detect urine at the catheter's tip. By utilizing a pump to expel the urine, the system achieves high-precision measurements, even capturing small urine volumes as low as 0.1 mL, which is particularly beneficial for pediatric patients. In addition, the system can detect blockages within the Foley catheter and tubing, providing alerts to medical staff who can quickly address these issues, ensuring a reliable measurement and uninterrupted urine flow.
Historically, many healthcare facilities have relied on visual inspections of urine collection bags every hour, often recording measurements manually. The automation and digital display of urine output by the FIZE kUO not only aid in the management of fluid balance for critically ill patients but also streamline workflows for healthcare providers, reducing their administrative burden. Furthermore, it minimizes the risk of human error during data entry and lowers the risks associated with managing infection-prone patients.
Addressing Critical Care Challenges
Asahi Kasei Medical is committed to embracing groundbreaking technology to resolve issues faced in critical care, aspiring to alleviate the workload of healthcare providers who are dedicated to patient safety. The FIZE kUO will follow the release of the TrachFlush, an endotracheal tube cuff inflator that began sales in 2024, introducing another valuable solution to the critical care landscape. As the company progresses, it aims to contribute to improved healthcare quality through the provision of innovative products and services, ultimately fostering a better medical environment.
Product Information
- - Product Name: FIZE kUO Automated Urine Monitoring System
- - Generic Name: Reusable Urine Flow Meter
- - Class Classification: Class I (General Medical Device)
- - Manufacturing and Sales Notification Number: 13B1X10145202501
About FIZE Medical Ltd.
Founded in 2014, FIZE Medical Ltd. is an Israeli medical device company dedicated to developing innovative technologies for real-time urine measurement. Their mission is to visualize renal function and optimize fluid management, ultimately improving the quality and efficiency of care for critically ill patients.
For more information, you can visit their official website: FIZE Medical
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.