

Sumitomo Bakelite and Olympus Marketing Form Sales Partnership for Innovative Hospital Stents
Innovative Collaboration for Healthcare Improvement
Sumitomo Bakelite Co., Ltd. has recently made headlines by forming a strategic sales partnership with Olympus Marketing Co., Ltd. This collaboration, set to kick off in early December 2025, aims to promote advanced medical devices, specifically stents designed to alleviate gastrointestinal blockages caused by malignant tumors. SB Kawasumi, a subsidiary of Sumitomo Bakelite and based in Kawasaki, Kanagawa, is at the forefront of this initiative and is responsible for the manufacturing of these cutting-edge products.
Products Overview
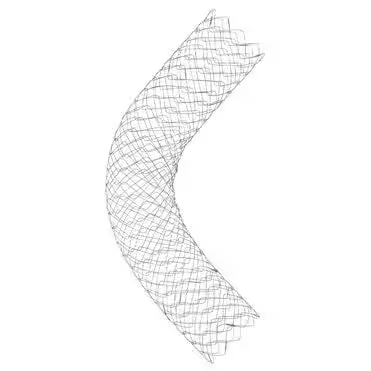
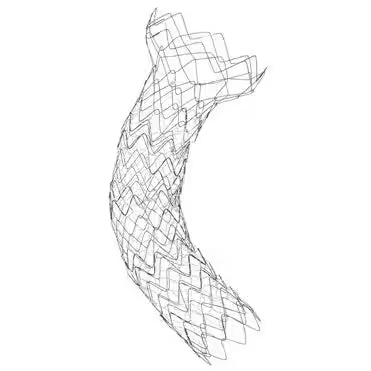
The collaboration focuses on the distribution of three types of stents: the Kawasumi Accordion Colonic Stent, the SR Colonic Stent, and the Spider Duodenum Stent. These stents are designed for patients suffering from obstructions in the colon or duodenum, providing an essential service in easing blockage symptoms through endoscopic placement.
Kawasumi Accordion Colonic Stent
- - Approval Number: 30100BZX00049000 (Class Ⅲ)
- - This innovative device features a silicone membrane covered by a reinforced fabric. This unique structure is designed to prevent re-obstruction caused by malignant tumor infiltration after the stent is inserted. Additionally, it helps mitigate damage to the tumor, ultimately contributing to improved patient quality of life (QOL).
SR Colonic Stent
- - Approval Number: 30600BZX00172000 (Class Ⅲ)
- - The SR Colonic Stent incorporates a mechanism that allows for the stepwise deployment of its three segments in any desired order during the insertion procedure. This design aims to facilitate precise positioning of the stent at the blockage site, enhancing the safety and reliability of the surgical technique, independent of the operator's skill level.
Spider Duodenum Stent
- - Approval Number: 30600BZX00015000 (Class Ⅲ)
- - Characterized by its hybrid structure combining metal and thread, this stent showcases superior flexibility and substantial expansion capability. Anticipated to conform well to bends within the digestive tract, it features a wide flare design that enhances adherence to the gastric wall near the pylorus, minimizing the risk of stent displacement. Such attributes are expected to improve food passage, thus significantly boosting patient QOL.
Future Commitment
Sumitomo Bakelite’s medical device division continues to evolve, focusing on surgical devices, perioperative devices, and endoscopic procedure devices. With this new partnership aimed at addressing critical medical needs, the company is dedicated to bringing innovative products to market quickly. This endeavor promises to improve not only the lives of patients but also the experiences of their families and healthcare providers.
Contact Information
For inquiries regarding sales or product details, please reach out to:
- - SB Kawasumi Co., Ltd.
Endoscopy Products Sales Promotion Division
Phone: 03-5462-4824
Contact Form
This partnership marks a significant step forward in the delivery of effective healthcare solutions by harnessing advanced technology to meet critical patient needs in Japan’s healthcare system.


Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.