

New Research Unveils Safety and Gene Expression of NMN in Dogs
Study on NMN Administration in Dogs: Insights and Findings
Introduction
In a groundbreaking study, Abe Yōandō Pharmaceutical, based in Tokyo, and Tottori University's Faculty of Agriculture have released findings on the safety of oral administration of nicotinamide mononucleotide (NMN) in dogs. This research marks an important step in veterinary medicines as it explores the impact of NMN on aging-related gene expression in canines.
Background
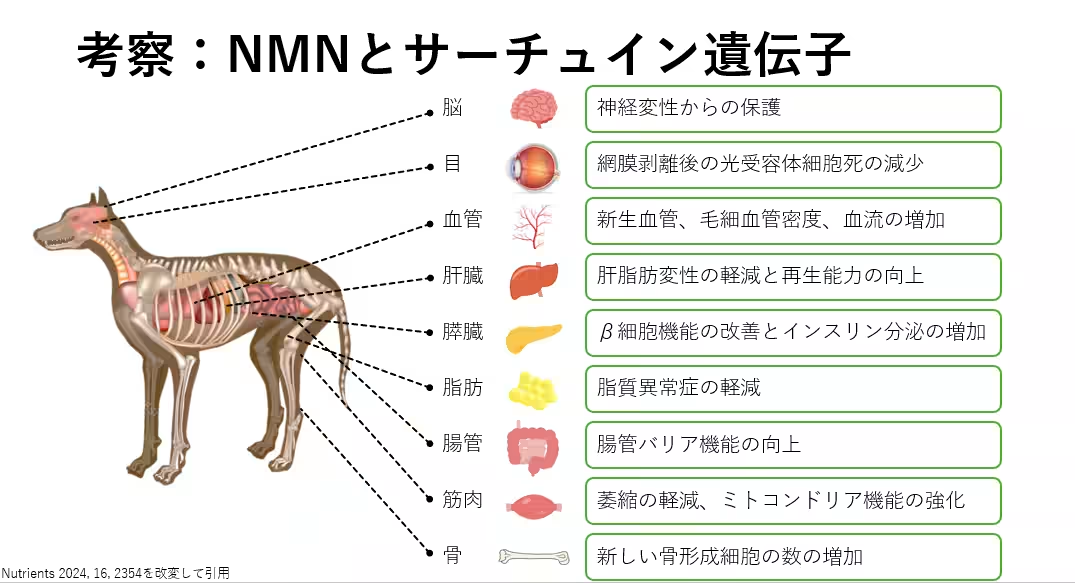
The Sirtuins gene family, including SIRT1, plays a crucial role in various biological processes such as aging, metabolism, DNA repair, and inflammation control. NMN serves as a precursor to NAD⁺, which may influence Sirtuin activity, suggesting its potential benefits in mitigating age-related issues not only in humans but also in pets such as dogs and cats.
This initiative comes at a time when research related to the safety and molecular effects of NMN in veterinary science remains limited. The study thus aims to bridge this knowledge gap, taking cues from existing human studies while focusing on the canine population affected similarly by aging
Overview of the Collaborative Research
Research Project Title
Investigating Aging-Related Changes in Dogs and Cats through Nicotinamide Mononucleotide
Study Duration
November 18, 2022 - March 31, 2025
Research Institutions Involved
- - Tottori University, Faculty of Agriculture, Clinical Veterinary Medicine
- - Abe Yōandō Pharmaceutical Co., Ltd.
Objective of the Study
To assess the safety of oral administration of NMN in dogs and cats, while gaining insight into its impact on aging-related genes, specifically the Sirtuin gene family.
Research Methods
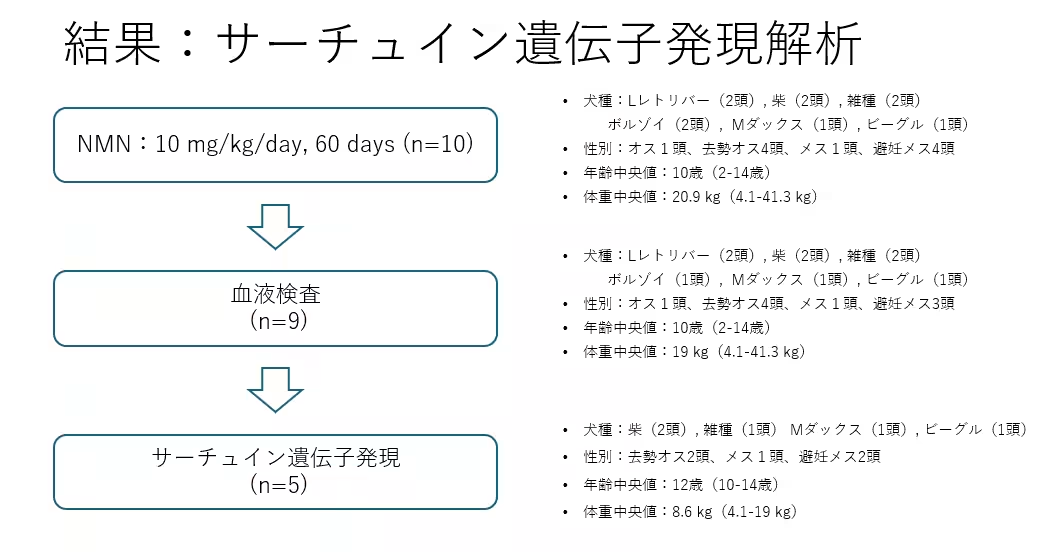
The trial involved ten healthy adult dogs, who were administered NMN orally over two months. The dosage was determined based on prior human studies. Comprehensive evaluations were performed, including blood tests (hematology and biochemistry) to ensure safety, as well as analysis of SIRT1 gene expression in blood samples.
Results of the Study
Out of the ten dogs, data was retrievable for nine, comprising one male, four neutered males, one female, and three spayed females, with a median age of 10 years and median weight of 19 kg. Throughout the two-month administration period, no significant adverse events or health issues were reported.
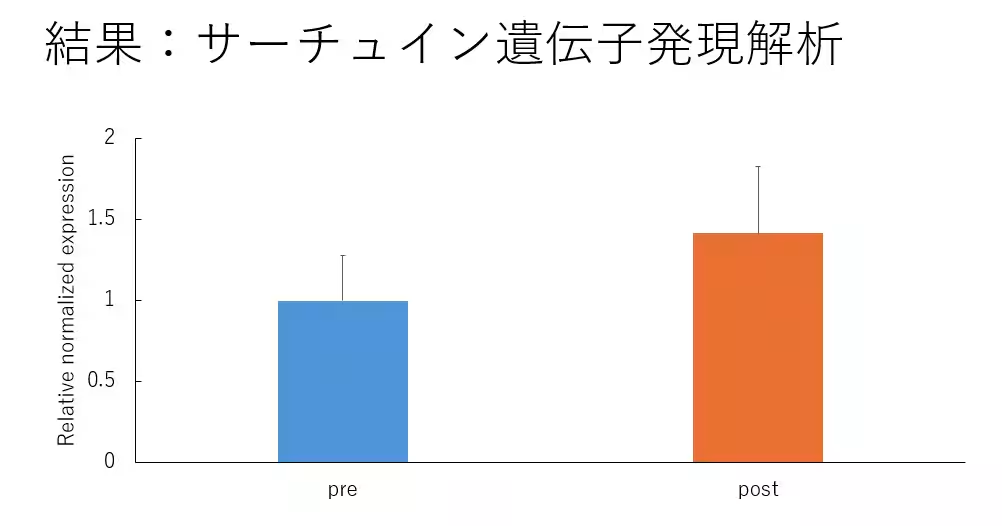
Additionally, extensive blood tests did not indicate any severe functional abnormalities in the organs. In five dogs from which sufficient genetic material was obtained for SIRT1 analysis, the median age was 12 years, and it was observed that there was an increase in the expression of the SIRT1 gene following NMN administration.
Discussion
The findings suggest that NMN administration might not pose major safety issues in dogs over the observed duration. The confirmation of increased SIRT1 gene expression encourages further exploration of NMN's biological effects related to aging.
This preliminary research establishes foundational data, although it does not specifically target any particular disease for prevention or treatment. The insights gained can serve as stepping stones for future investigative endeavors in the field.
Future Prospects
Abe Yōandō Pharmaceutical commits to continuing its science-based product development. Ongoing research will further unveil the potential of NMN, aiming to support healthier, fulfilling lives for both pets and their owners.
Conclusion
The study provides fundamental data regarding the safety and molecular changes associated with NMN administration in dogs. These results are poised to contribute significantly to the veterinary field's understanding of NMN, while research involving feline subjects continues, with no considerable safety concerns noted so far.
About Abe Yōandō Pharmaceutical
Since its establishment in 1731, Abe Yōandō Pharmaceutical has been a pioneer in NMN research, leading to legislative changes that recognize NMN as a food supplement. With a commitment to high-quality product development, the company remains dedicated to supporting the health and well-being of its customers.
For more information, visit their Official Website.



Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.