

Identifying Potential Violations of Pharmaceutical Advertising Laws in Japan: Regular Survey Results
In a recent survey conducted by REGAL CORE, a prominent company specializing in pharmaceutical law inspections, various advertisements were analyzed to assess compliance with Japan's Pharmaceutical and Medical Device Act (PMDA) and the Act Against Unjustifiable Premiums and Misleading Representations. Given the intricate nature of these laws, ensuring that advertisements do not mislead consumers about the effectiveness of health-related products is paramount.
From December 2025 to January 2026, REGAL CORE scrutinized multiple web media sources, paying particular attention to article landing pages that contained promotional content. The aim was to identify any advertising expressions that could potentially contravene existing regulations. The findings suggest troubling trends with several ads displaying statements that may mislead consumers about health benefits.
The survey revealed recurring issues, similar to previous assessments, including a variety of advertisements that suggest illicit health benefits. Here are specific examples identified:
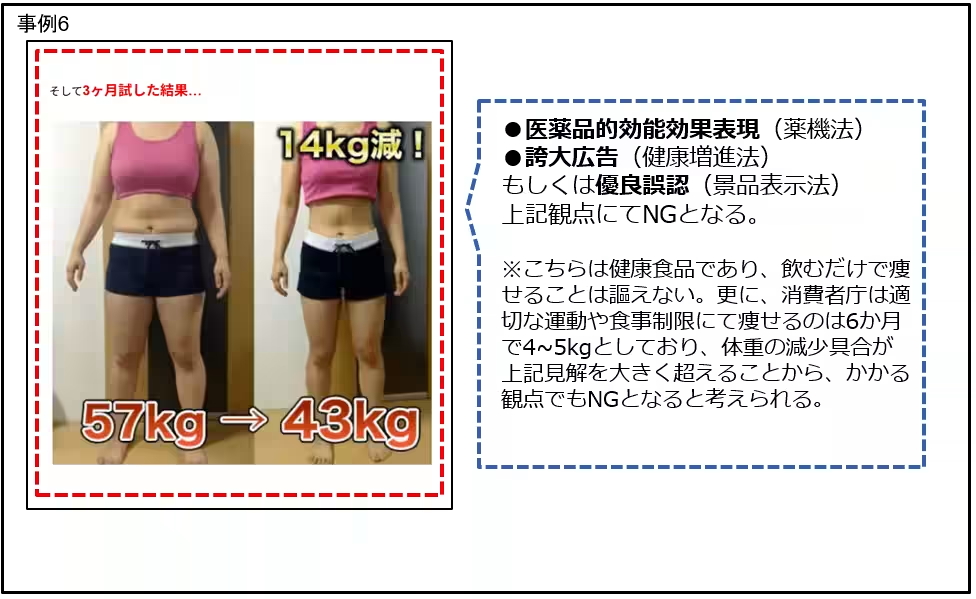
- Language implying effortless weight loss, such as "guaranteed weight loss" or encouraging consumers to think they can lose significant weight in an unrealistically short period.
- Suggestive expressions that imply improvements in male functions simply by consumption seem to cross the line.
- Ads offering dubious promises related to digestive efficacy or rapid weight reduction without lifestyle changes also raised alarms.
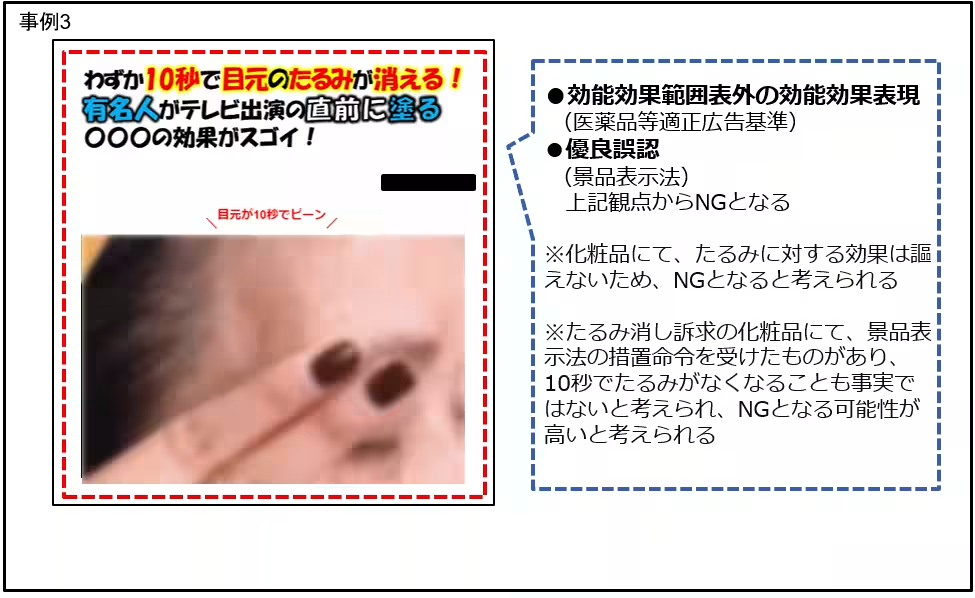
- Ads claiming dramatic skin improvement or wrinkle reduction without appropriate approvals were frequently noted, particularly those showcasing before-and-after images.
The results of this ongoing investigation emphasize the need for ongoing vigilance against misleading advertising practices. While some advertisers have taken steps to rectify their claims, it's imperative that all stakeholders in the health and cosmetic sectors remain compliant with advertising regulations. Failure to do so can not only lead to consumer deception but also potential legal repercussions.
Since August 2022, REGAL CORE has consistently refined its survey methodologies and interpretations, ensuring timely updates on advertising compliance. Regular reports detailing these findings serve as reminders to businesses to remain diligent in their marketing practices. Ultimately, consumer protection remains the top priority, and ongoing scrutiny will help mitigate misleading claims.
In conclusion, the investigation into advertising practices relating to pharmaceuticals and health products continues to reveal concerning trends. Businesses should take this opportunity to review and adjust their marketing strategies to ensure compliance with Japanese law, thereby fostering transparency and trust with consumers.




Survey Overview
From December 2025 to January 2026, REGAL CORE scrutinized multiple web media sources, paying particular attention to article landing pages that contained promotional content. The aim was to identify any advertising expressions that could potentially contravene existing regulations. The findings suggest troubling trends with several ads displaying statements that may mislead consumers about health benefits.
Findings on Problematic Advertising Expressions
The survey revealed recurring issues, similar to previous assessments, including a variety of advertisements that suggest illicit health benefits. Here are specific examples identified:
Health Products
- - Many ads appear to claim pharmaceutical effects, which is a violation under PMDA. Examples include:
- Language implying effortless weight loss, such as "guaranteed weight loss" or encouraging consumers to think they can lose significant weight in an unrealistically short period.
- Suggestive expressions that imply improvements in male functions simply by consumption seem to cross the line.
Functional Foods
- - Additional concerns were raised regarding claims extending beyond the approved functional benefits of products, including:
- Ads offering dubious promises related to digestive efficacy or rapid weight reduction without lifestyle changes also raised alarms.
Cosmetics
- - Advertisements for skincare products showed significant violations, making claims that imply:
- Ads claiming dramatic skin improvement or wrinkle reduction without appropriate approvals were frequently noted, particularly those showcasing before-and-after images.
- - Even products such as hair growth treatments or eyelash serums were found making unapproved claims, suggesting effectiveness without substantiation.
Regulatory Implications and Compliance
The results of this ongoing investigation emphasize the need for ongoing vigilance against misleading advertising practices. While some advertisers have taken steps to rectify their claims, it's imperative that all stakeholders in the health and cosmetic sectors remain compliant with advertising regulations. Failure to do so can not only lead to consumer deception but also potential legal repercussions.
Continuous Monitoring and Future Updates
Since August 2022, REGAL CORE has consistently refined its survey methodologies and interpretations, ensuring timely updates on advertising compliance. Regular reports detailing these findings serve as reminders to businesses to remain diligent in their marketing practices. Ultimately, consumer protection remains the top priority, and ongoing scrutiny will help mitigate misleading claims.
In conclusion, the investigation into advertising practices relating to pharmaceuticals and health products continues to reveal concerning trends. Businesses should take this opportunity to review and adjust their marketing strategies to ensure compliance with Japanese law, thereby fostering transparency and trust with consumers.






Topics Consumer Products & Retail)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.