
Key Discovery in Alzheimer's Disease: A Reversible Precursor for Tau Aggregation Prevention
A New Breakthrough in Alzheimer's Disease Research
Introduction
Alzheimer's disease has increasingly become a significant concern worldwide, characterized by the gradual decline of memory and cognitive functions. It is well-known that this condition is largely associated with the abnormal aggregation of tau proteins, which ultimately lead to neurodegeneration. Despite deep investigations, the precise mechanisms through which tau aggregation begins, especially in its early stages, have been minimally explored until now.
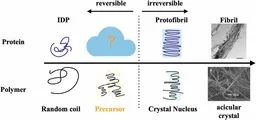
A research team from Tokyo Metropolitan University, led by PhD candidate Tomomi Takahashi and Professor Rei Kurita, in collaboration with experts from the Tokyo Medical and Dental University and the University of Tokyo, has made a pivotal discovery. They found that tau proteins form enormous precursor clusters before transitioning into fibrillation. These clusters, measuring several tens of nanometers in size, possess a reversible structure, distinguishing them from the rigid fibrils typically associated with tau aggregation.
Key Findings
1. Formation of Precursor Clusters: It has been established that prior to fibrillization, tau proteins assemble into large precursor clusters. This is a significant revelation in understanding the initial stages of tau aggregation.
2. Reversible Nature: The discovered precursor clusters can be dismantled, which marks a considerable difference from the non-reversible fibrillation process.
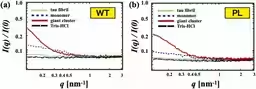
3. Prevention of Fibrillation: By introducing sodium chloride (NaCl), the research demonstrated that these precursor clusters could be broken apart, leading to a marked delay in the fibrillization process.
4. Potential for Treatment: Targeting these precursor clusters could open up avenues for new therapeutic strategies aimed at delaying the onset and progression of Alzheimer's disease.
Background of the Study
Alzheimer’s disease manifests widely as a neurodegenerative disorder where memory and cognitive abilities decline progressively. Two hallmark changes are observed in patients’ brains: the formation of amyloid plaques and neurofibrillary tangles created by tau protein aggregation. Historically, much of the research has focused on amyloid-beta, investigating its aggregation mechanisms and the development of drug targets against it. However, the fibrillization of tau has been noted to occur even before the full manifestation of dementia, emphasizing the need for direct therapeutic strategies against tau.
Tau proteins, classified as intrinsically disordered proteins, do not maintain a stable three-dimensional structure yet carry out crucial cellular functions. The transformation of these proteins into ordered fibrils presents a significant scientific challenge that has yet to be fully understood from a physical standpoint.
Research Methodology
In this study, the research group adopted a polyphysics perspective to better understand tau aggregation. Drawing upon research in polymer crystals, they explored how disordered chain-like molecules transition through precursor states, which ultimately influence their final crystallized structures. They hypothesized that similar principles could be applied to the fibrillation of tau proteins.
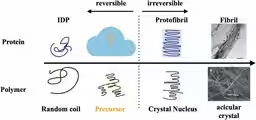
Utilizing small-angle X-ray scattering (SAXS), the team investigated tau proteins that had been treated with heparin. The experiments revealed the formation of these large precursor clusters, which displayed a dynamic nature—continuously forming and disassembling rather than being rigidly structured like amyloid fibrils.
More importantly, experimenting with sodium chloride (NaCl) further illustrated that inducing disruptions in this dynamic structure significantly delayed the fibrillation of tau proteins. This striking finding has crucial implications for future therapies aimed at mitigating the effects of Alzheimer's.
Implications and Future Directions
The discovery of these precursor clusters as a significant midpoint in the fibrillation process presents a new, tractable target for therapeutic intervention. The research shifts focus from addressing completed tau fibrils to the reversible precursor structures that may guide the pathogenic process in neurodegenerative diseases.
By further understanding tau aggregation, the findings may also extend beyond Alzheimer’s disease, potentially impacting treatment approaches for other protein misfolding disorders, such as Parkinson’s disease and amyotrophic lateral sclerosis (ALS). As research progresses, it promises to bolster the intricate link between tau proteins and neurodegeneration, paving the way for novel non-invasive therapeutic approaches that could significantly alter the outlook on disease intervention.
Conclusion
This groundbreaking study adds a crucial piece to the Alzheimer's puzzle by illustrating the potential for disrupting tau protein aggregation through precursor clustering. As research continues in this field, the hope for effective preventive measures and treatments for Alzheimer's disease becomes increasingly tangible.
Reference
The research findings were published on October 8 in the journal Neuroscience Research. Full details can be viewed in the article titled "Hindering Tau Fibrillization by Disrupting Transient Precursor Clusters." DOI: 10.1016/j.neures.2025.104968.
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.