

Urgent Warning Regarding Possible Counterfeit Medical Devices on Amazon Japan
Urgent Warning Regarding Counterfeit Medical Devices
A recent ruling by the Tokyo District Court on April 25, 2025, has shed light on the ongoing issue of counterfeit medical devices available on Amazon Japan. Presided over by Judge Yuko Shintani, the court recognized Amazon Japan's breach of duties, ordering the company to compensate the plaintiff, Excel Plan Co., Ltd. Despite this legal acknowledgment, Amazon and the regulatory authorities continue to neglect the circulation of potentially harmful unapproved medical devices, failing to issue recalls or inform consumers about the risks.
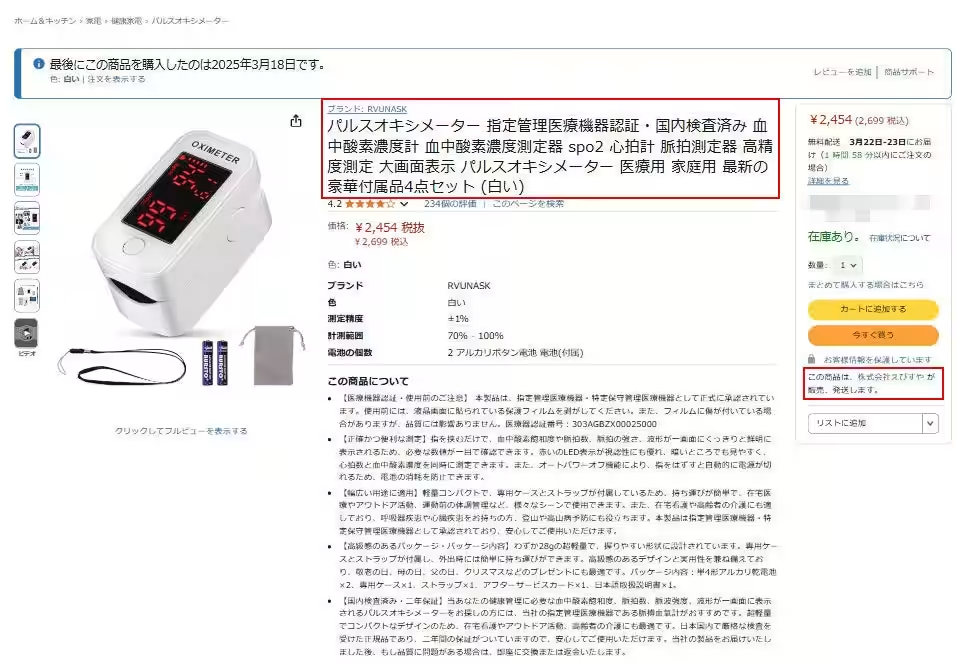
Since the COVID-19 pandemic, a surge in unapproved medical devices has been reported on Amazon. Specifically, consumers who have purchased pulse oximeters from brands like the Pulxy Plus series, MyCare OX series, and MediPal Ace from any seller other than the authorized shop “Pure Clean,” are urged to check their order history. These products may originate from Chinese sellers and carry the risk of being unapproved medical devices. Shoppers are strongly advised to discontinue use and reach out to us immediately.
Important Warning: This communication aims to inform consumers misled into purchasing counterfeit medical devices on how to proceed. We do not handle refunds or recalls on behalf of consumers.
Examples of Counterfeit Medical Devices
1. Example 1 of counterfeit medical devices
2. Example 2 of counterfeit medical devices
3. Example 3 of counterfeit medical devices
4. Example 4 of counterfeit medical devices
Visual Comparison:
- - The legitimate product shows the medical device certification number, serial number, and manufacturer details on the battery cover (left). In contrast, the unapproved medical device lacks any such markings (right).
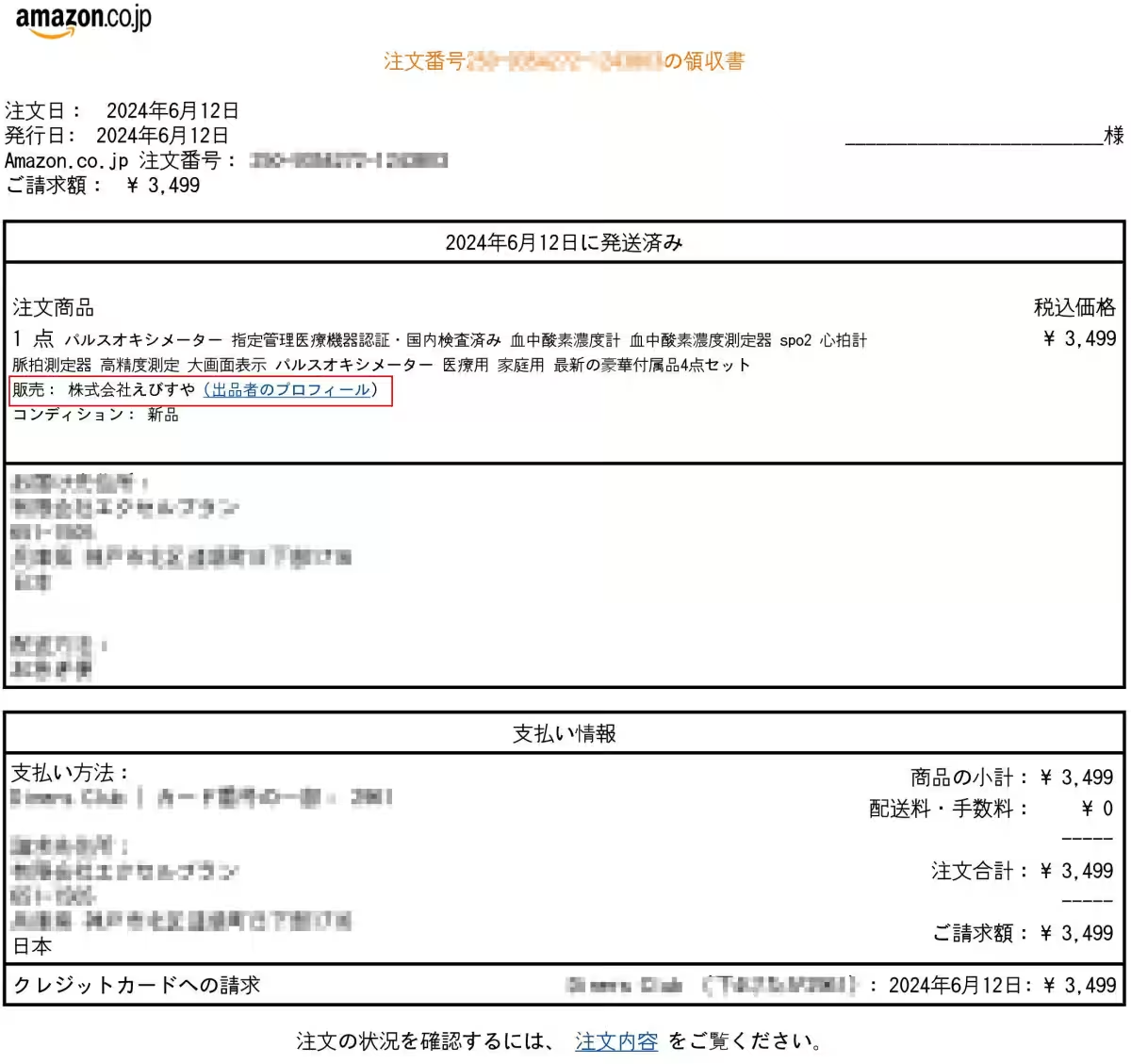
Additionally, a pulse oximeter sold by Ebisuya Co., Ltd. falsely advertised medical device certification along with its listing, claiming compliance with domestic testing standards. Unfortunately, the certification numbers provided were discovered to belong to another company, and the business address listed under the Specified Commercial Transaction Act shows no substantial operations. Moreover, the medical device sales license number confirmed by the Miyazaki City Health Office was found to be invalid.
Packaging Information: Internal packaging of unapproved medical devices and its respective battery cover markings are displayed here.
Business Information of Ebisuya Co., Ltd.
- - Seller: Ebisuya Co., Ltd.
- - Customer Support Phone: +17726773343
- - Address: 3274-37 Komatsu, Miyazaki City, Miyazaki Prefecture, 880-2103, Japan
- - Responsible Manager: Takefumi Ito
- - Store Name: Ebisuya Co., Ltd.
- - License Number: Miyazaki No. 129
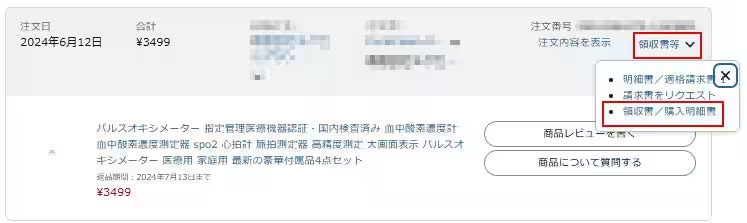
If you suspect you have purchased these items, please note that the seller's product pages have been removed. You are encouraged to verify your purchase by checking your Amazon order history and keeping any related receipts or purchase statements for reference.
Immediate Action Required: Discontinue use of the suspected products and contact us as soon as possible.
Note: This guide serves to prevent further sales of counterfeit products and assist consumers misled into purchasing them, but we do not handle refunds or returns on behalf of consumers.









Topics Consumer Products & Retail)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.