

New Treatment Recommendations for High-Grade Osteosarcoma in Pediatric and AYA Patients
New Treatment Recommendations for High-Grade Osteosarcoma in Pediatric and AYA Patients
Recent research by the Japan Clinical Oncology Group (JCOG) has important implications for the treatment of high-grade osteosarcoma, particularly in young patients. The study presents new findings indicating that for pediatric and AYA (Adolescents and Young Adults) patients diagnosed with high-grade osteosarcoma, maintaining a three-drug regimen known as MAP therapy might be more beneficial than introducing a fourth drug in cases where preoperative chemotherapy is not effective.
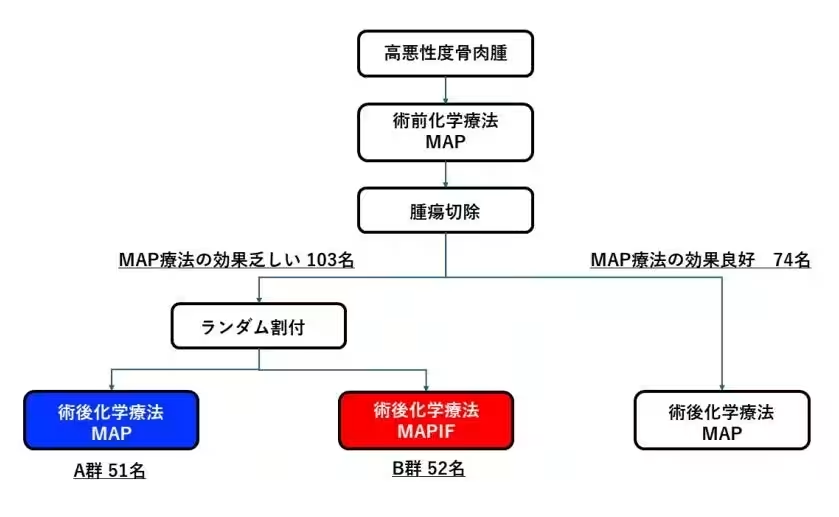
Traditionally, the standard approach for managing non-metastatic high-grade osteosarcoma has involved administering a combination of three chemotherapeutic agents: Methotrexate (M), Doxorubicin (A), and Cisplatin (P). This regimen, known as MAP therapy, is widely accepted as a baseline treatment protocol. However, when the efficacy of the preoperative MAP therapy is deemed insufficient, oncologists often opt for a more aggressive four-drug regimen called MAPIF, which incorporates Ifosfamide (IF).
While the intent behind the MAPIF approach is to enhance treatment outcomes, the JCOG study, involving 34 institutions across the nation, sought to clarify whether the addition of Ifosfamide truly offers a significant benefit. Concerns had arisen regarding the potential for this added chemotherapy to result in prolonged treatment durations and cause unforeseen side effects.
The results were striking. After a randomized controlled trial comparing post-operative MAP therapy with MAPIF in patients who did not respond well to preoperative chemotherapy, the findings demonstrated that MAPIF did not contribute to improved survival times. In fact, increasing the treatment complexity with the addition of Ifosfamide presented greater risks of adverse side effects.
Consequently, the research supports the conclusion that for pediatric and AYA patients with non-metastatic high-grade osteosarcoma, retaining the simpler three-drug MAP therapy, even after an ineffective initial chemotherapy response, is the preferable route. This revelation represents a significant shift in standard treatment protocols and has garnered international recognition, culminating in its publication in the prestigious medical journal, Journal of Clinical Oncology.
Dr. Hiroaki Hiraga and his collaborators highlight that these findings align with ongoing efforts to refine treatment standards based on rigorous scientific evidence, ultimately aiming to establish optimal care pathways for young patients facing the challenges posed by osteosarcoma.
Moving forward, JCOG plans to continue its efforts in clinical trials, committed to enhancing the quality of cancer care for patients. This dedication not only supports the advancement of effective treatment standards in oncology but also reflects an ongoing commitment to fostering innovation within cancer research.
As the medical community adjusts to these new insights, the hope is that the treatment of osteosarcoma will improve, ensuring better outcomes for young patients across the globe. The future of cancer care looks promising, with JCOG set to lead the charge in establishing protocols based on conclusive evidence, tailored specifically for the needs of pediatric and adolescent communities.
For more detailed information on the study and its implications, visit JCOG's official site or the Journal of Clinical Oncology.





Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.