

Developing Innovative Technology to Extract Potassium from Seawater: A Leap Towards Domestic Fertilizer Production
New Technology to Extract Potassium from Seawater
In a significant breakthrough, the National Institute of Advanced Industrial Science and Technology (AIST) has developed a pioneering technology aimed at selectively recovering potassium from seawater. This innovative method is expected to facilitate the stable production of potassium—an essential nutrient for plant growth—domestically. Currently, Japan heavily relies on imported fertilizers, making its agricultural supply chain vulnerable to geopolitical risks and external conflicts.
The Importance of Potassium in Agriculture
Potassium (K), alongside nitrogen (N) and phosphorus (P), constitutes one of the three vital nutrients required for plant development. Notably, Japan's dependence on fertilizer imports raises concerns about food security and the potential rise in fertilizer prices due to geopolitical tensions. Despite the abundance of potassium in seawater, with concentrations as low as 0.04%, effective utilization has been previously hampered by its low concentration, coupled with the presence of competing sodium ions, which are chemically similar.
Currently, methods to extract potassium from seawater primarily involve the production of salt, resulting in limited output. If a direct extraction method could be established, there would be a possibility to meet domestic potassium demands efficiently.
The Innovative Approach
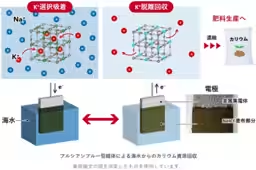
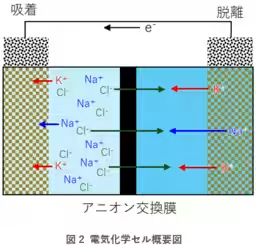
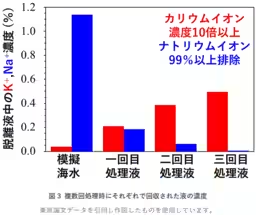
Researchers at AIST, including Masakuni Yamaguchi, Takeo Tomiyama, and Tohru Kawamoto, have developed a technique using a Prussian blue-type complex applied to electrodes. By electrochemically controlling these electrodes, the method enables the selective adsorption and desorption of potassium ions from seawater. The team demonstrated that after three cycles of this process, sodium ions could be eliminated by over 99%, achieving a tenfold concentration of potassium ions in the solution extracted from simulated seawater.
This technology opens up pathways to produce the potassium resources necessary for crop growth in a stable domestic structure.
Addressing the Challenges of Low Concentration
One of the main difficulties addressed by AIST was the extremely low concentration of potassium ions in seawater. To overcome this, the Prussian blue-type complex was used not only for its historical use in various applications but also for its selectivity towards potassium ions over competing sodium ions. The researchers modified the properties of the Prussian blue complex to ensure efficiency in potassium recovery by identifying an optimal metal combination for the complex. This involved using nickel and iron variants of the complex that exhibited high selectivity for potassium ions.
Further optimizations included the development of electrodes that could handle the specific conditions of seawater. The electrodes had to balance between having a larger surface area for potassium adsorption and visiting the limitations imposed by liquid diffusion within them. A porous metal collector was utilized to enhance performance, resulting in a tenfold increase in adsorption capacity compared to traditional methods.
Future Directions
Looking ahead, the team at AIST aims to enhance the concentration of the potassium solution to levels viable for use in liquid fertilizers. Additionally, efforts will continue towards simplifying the processing technique to enable practical applications, including utilizing real seawater for tests and developing cost-effective solidification methods for fertilizers. These advancements promise to not only enhance domestic fertilizer production but also fortify Japan's agricultural sector against potential future disruptions.
The findings from this research have been documented in the journal Water Research and are a testament to AIST's commitment to fostering innovative solutions for agricultural sustainability and food security in Japan.
Conclusion
AIST's technology represents a critical step forward in addressing Japan's fertilizer production challenges, demonstrating the potential of seawater as a sustainable resource. By harnessing such innovative methods, Japan could significantly reduce its dependence on imported fertilizers, contributing toward a more self-reliant agricultural framework.

Topics Consumer Products & Retail)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.