

Innovative Heart Disease Treatment for Dogs Secures Patents in Korea and China
New Treatment for Canine Heart Diseases
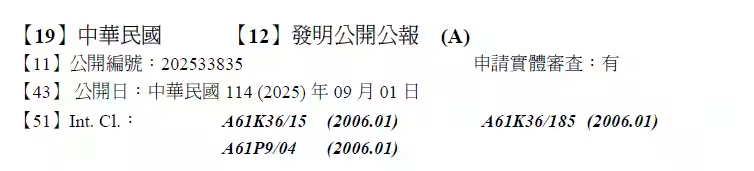
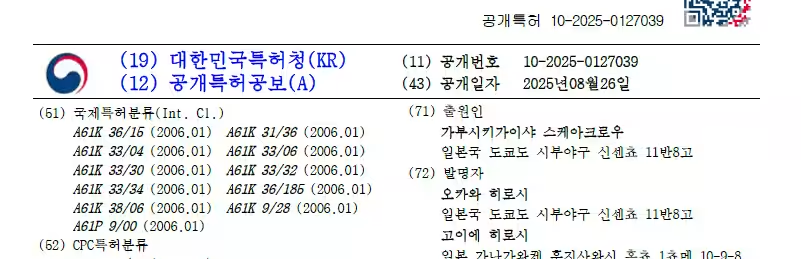
In a significant advancement for pet health, Scarecrow Inc., based in Shibuya, Tokyo, has recently obtained patents for their innovative heart disease treatment for dogs, known as the Atrial Natriuretic Peptide (ANP) lowering agent. This product, including both therapeutic and preventive aspects, is now protected under patent law in Korea and Taiwan, with aspirations for widespread distribution. The company's mission is to empower dog owners worldwide to easily maintain their furry friends' heart health through food and supplements.
Understanding Canine Heart Disease in Japan
With approximately 6.8 million pet dogs in Japan (as reported by the Pet Food Association in 2024), a considerable proportion consists of small breeds. These small breeds are genetically predisposed to various heart conditions, particularly Mitral Valve Insufficiency, which ranks as the second leading cause of death among older dogs (according to Anicom HD in 2024). This alarming trend highlights the urgent need for effective treatments for canine heart diseases.
A key indicator of heart disease severity in dogs is the level of Atrial Natriuretic Peptide (ANP), a hormone synthesized and stored in the heart's atrial chambers, which can be measured through blood tests. It serves as a vital biomarker for canine heart health, shedding light on the pet's current cardiovascular status. Recent studies have demonstrated that a complex of polyphenols can alleviate cardiac stress and lower ANP levels effectively, leading to the successful acquisition of the patent.
Trial Studies and Results
In the pursuit of this patent, clinical trials were conducted across 15 veterinary hospitals in Japan, involving 27 dogs diagnosed with Mitral Valve Insufficiency. These trials included a 30-day administration of the polyphenol complex alongside the ongoing cardiac medication. Blood tests before the trial commenced and after 30 days showed significant reductions in ANP levels, indicating improvement in heart function. Moreover, notable enhancements in the dogs' respiratory comfort were observed during this period, as presented at the Annual Conference of Veterinary Clinical Medicine in October 2023.
Inventive Details
- - Inventive Title: Atrial Natriuretic Peptide Lowering Therapeutic and Preventive Agent and Production Method
- - Registration Number: P7542838
- - Registration Date: August 23, 2024
- - Patentee: Scarecrow Inc.
- - Inventors: Hiroshi Okawa, Hiroshi Koiwa
- - Application Date: February 15, 2024
The Role of Polyphenol Complex
The key ingredient in the polyphenol complex comes from the bark of French maritime pine exclusively cultivated in southwestern France. This water-soluble natural food source is identified as a safe and highly physiologically active component. Extensive research has led to the accumulation of data from over 800 studies worldwide, underscoring its efficacy in supporting canine heart health.
Guidelines by the American College of Veterinary Internal Medicine (ACVIM) categorize Mitral Valve Insufficiency into stages ranging from A to D, where stage A indicates mild sickness and stage D severe. Unfortunately, many owners find themselves in distress when their dogs are aware of heart issues but fall short of immediate medication, especially in the earlier stages.
This is where the polyphenol complex can intervene, providing pet owners with a proactive solution. Introducing this complex at early stages, such as A or B1, can support heart health and contribute to their dog's overall vitality from a young age.
About Scarecrow Inc.
Scarecrow Inc. specializes in researching, developing, and marketing animal supplements. Committed to using natural ingredients tailored to specific health concerns, the company has established a presence in over 4,000 domestic hospitals and facilities, as well as 22 countries worldwide.
Company Overview:
- - Company Name: Scarecrow Inc.
- - Headquarters: 2nd Floor, Umezan Building, 11-8 Shinsen-cho, Shibuya, Tokyo
- - Representative: Hiroshi Okawa
- - Business Focus: Research, development, and marketing of supplements for small animals
- - Established: March 30, 2004
- - Website: Scarecrow Inc.
This press release has undergone legal review.


Topics Other)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.