

Regulatory Concerns on Advertisements: Recent Findings from REGAL CORE's Research
REGAL CORE's Ongoing Research on Misleading Advertisements
In a recent study conducted from November to December 2025, REGAL CORE, a Tokyo-based firm specializing in Pharmaceutical Affairs Law checks, investigated advertisements that potentially violate Japanese laws. Their findings raise significant concerns about compliance practices in online advertising, particularly those that could mislead consumers.
Methodology of the Study
REGAL CORE undertakes a systematic approach to gather advertisements appearing on multiple web media platforms, focusing primarily on landing pages of recommended products. The evaluation process involves scrutinizing these entries to identify expressions that could be misleading or legally non-compliant based on the Pharmaceutical Affairs Law and the Law on Specified Commercial Transactions.
Key Findings of the Investigation
Despite previous warnings, the latest round of checks has revealed continuing issues with advertisements that feature expressions likely to contravene legal standards. The summary of problematic expressions identified includes those that imply drug-like efficacy for health foods or those that misrepresent cosmetic products.
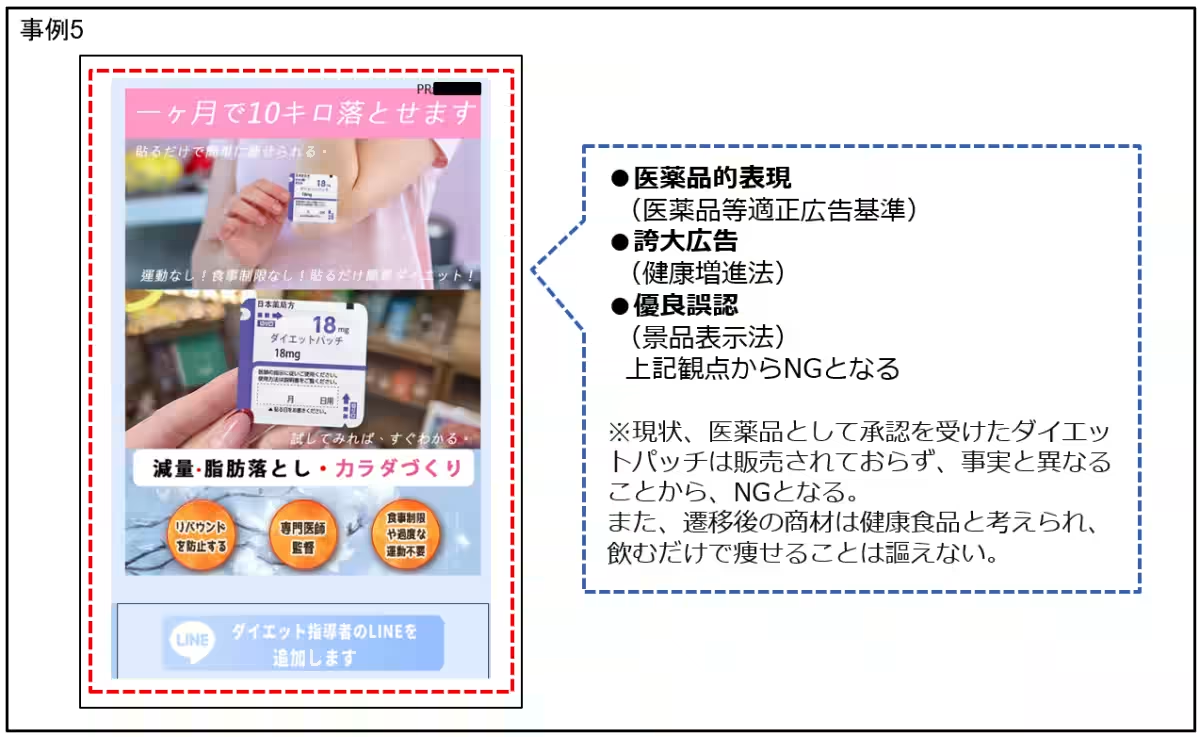
Health Food Advertisements
Researchers unearthed alarming usages such as claims suggesting that certain products could affect female hormones or provide hyperbolic outcomes like significant weight loss or enhanced male performance. Specific examples included:
- - Claims indicating unnatural benefits, such as 'achieving full and firm breasts' or promises for 'improved metabolism with just this drink.' Such assertions diverge significantly from the allowable advertising claims for health-related products, violating both health promotion standards and the quality representation law.
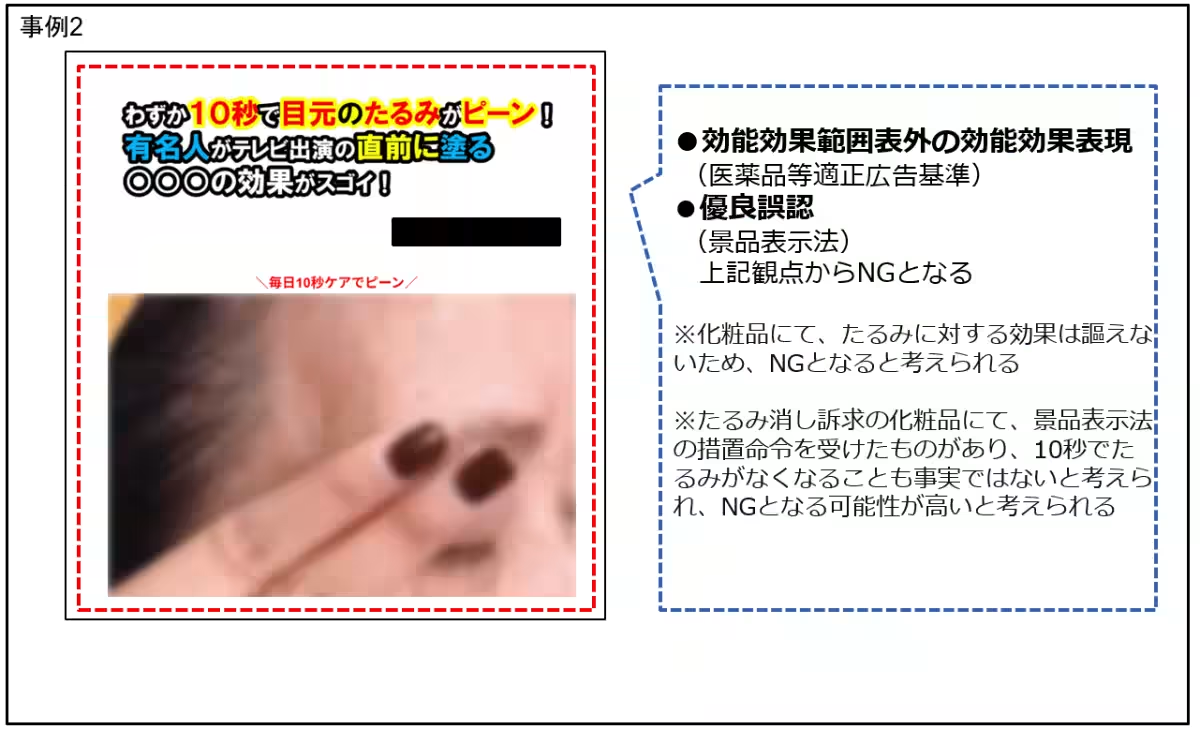
Cosmetic Product Claims
In the cosmetics sector, multiple advertisements exaggerated product benefits that do not comply with legal guidelines. Examples include:
- - Claims regarding the total removal of scars or wrinkles as well as promises for transformative effects from common skincare products without adequate scientific backing. Such misleading claims can lead consumers to have unrealistic expectations.
Implications for Consumers and Advertisers
The presence of these misleading claims poses a dual risk: undermining consumer trust and leading businesses to potential legal troubles. Consumers must recognize that while health and beauty products promise significant results, the efficacy of these claims is often overstated. Advertisers, on the other hand, are encouraged to follow established guidelines closely to avoid repercussions.
Recommendations for Ethical Advertising
REGAL CORE emphasizes the need for clear, honest communication. Companies should refrain from making unverifiable claims and focus instead on providing products backed by legitimate research. Regular reviews of advertising content against current laws are also crucial to ensuring compliance.
The Path Forward
Many businesses have already taken steps to amend their advertising strategies following past recommendations. Continuous adaptation and feedback will play a fundamental role in aligning with legal standards. As REGAL CORE continues its investigations, it remains committed to updating its findings to reflect current trends and urges all stakeholders to promote transparency and fairness in advertising.
In summary, REGAL CORE's ongoing vigilance serves as a necessary reminder of the importance of regulatory compliance in marketing. The reported findings should prompt both consumers and marketers to contemplate their respective roles in maintaining integrity within the marketplace. As regulatory landscapes evolve, so too must the standards of communication in advertisement practices.




Topics Consumer Products & Retail)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.