
Exploring the Hierarchical Structures of Water Confined in Nanocrystals Reveals Novel Pre-Melting State
Exploring the Hierarchical Structures of Water Confined in Nanocrystals Reveals Novel Pre-Melting State
A recent study conducted by a joint research team from Tokyo University of Science and Kanazawa University has made significant strides in understanding the intricacies of water confined within nanocrystalline structures using solid 2H NMR techniques. This research reveals the hierarchical nature of water in confined spaces, uncovering a previously unknown pre-melting state that holds promising implications for various scientific fields, including biochemistry and energy materials.
Background of the Study
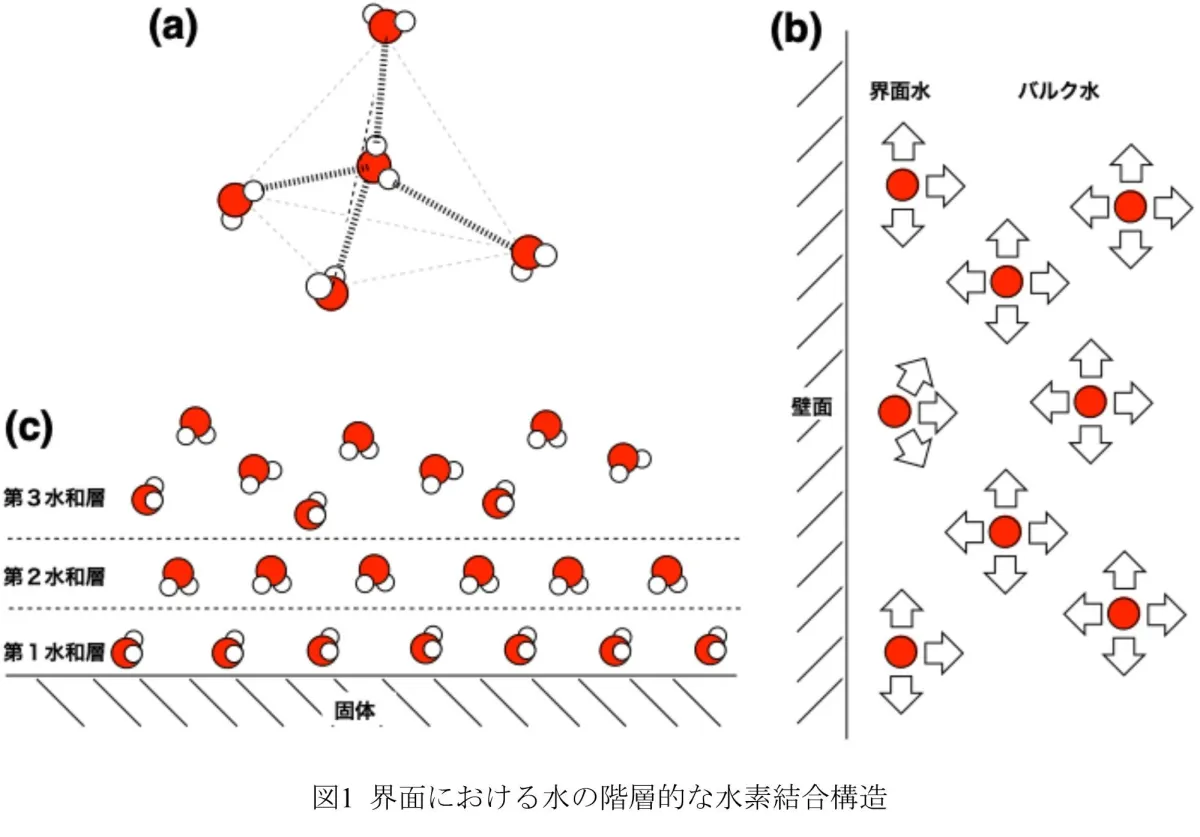
Water, in its various states, exhibits unique properties at the molecular level, particularly when confined within nanoscale structures. Traditional bulk water exhibits stable tetrahedral hydrogen bonding, but the interactions at solid-liquid interfaces create complexities that are challenging to investigate due to the inherent irregularities. This research focuses on one-dimensional nanocrystalline pores synthesized to study the confined water's various properties more accurately.
The joint research team, led by Professor Makoto Tadokoro and Lecturer Fumiya Kobayashi at the Department of Chemistry at the Tokyo University of Science, and graduate student Tomoya Namiki, collaborated with Kanazawa University to analyze the behavior of deuterated water clusters (D2O) within these confined environments.
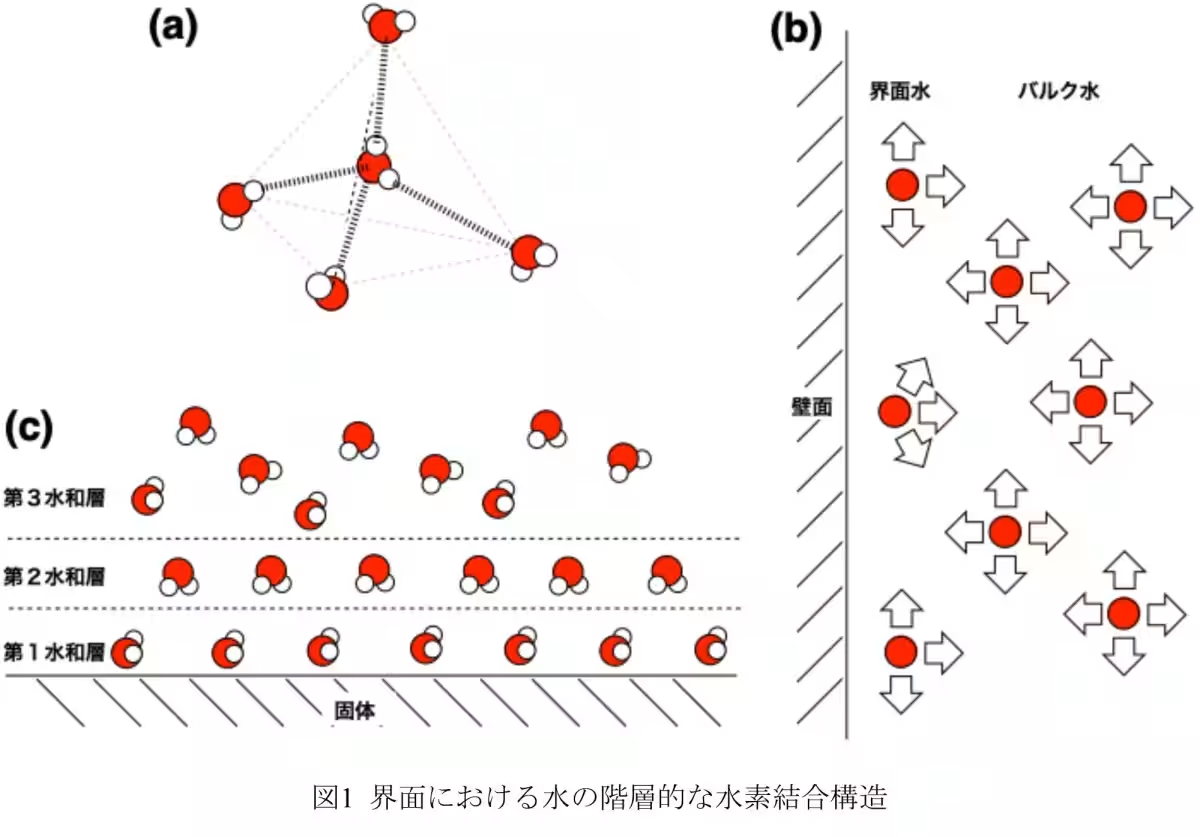
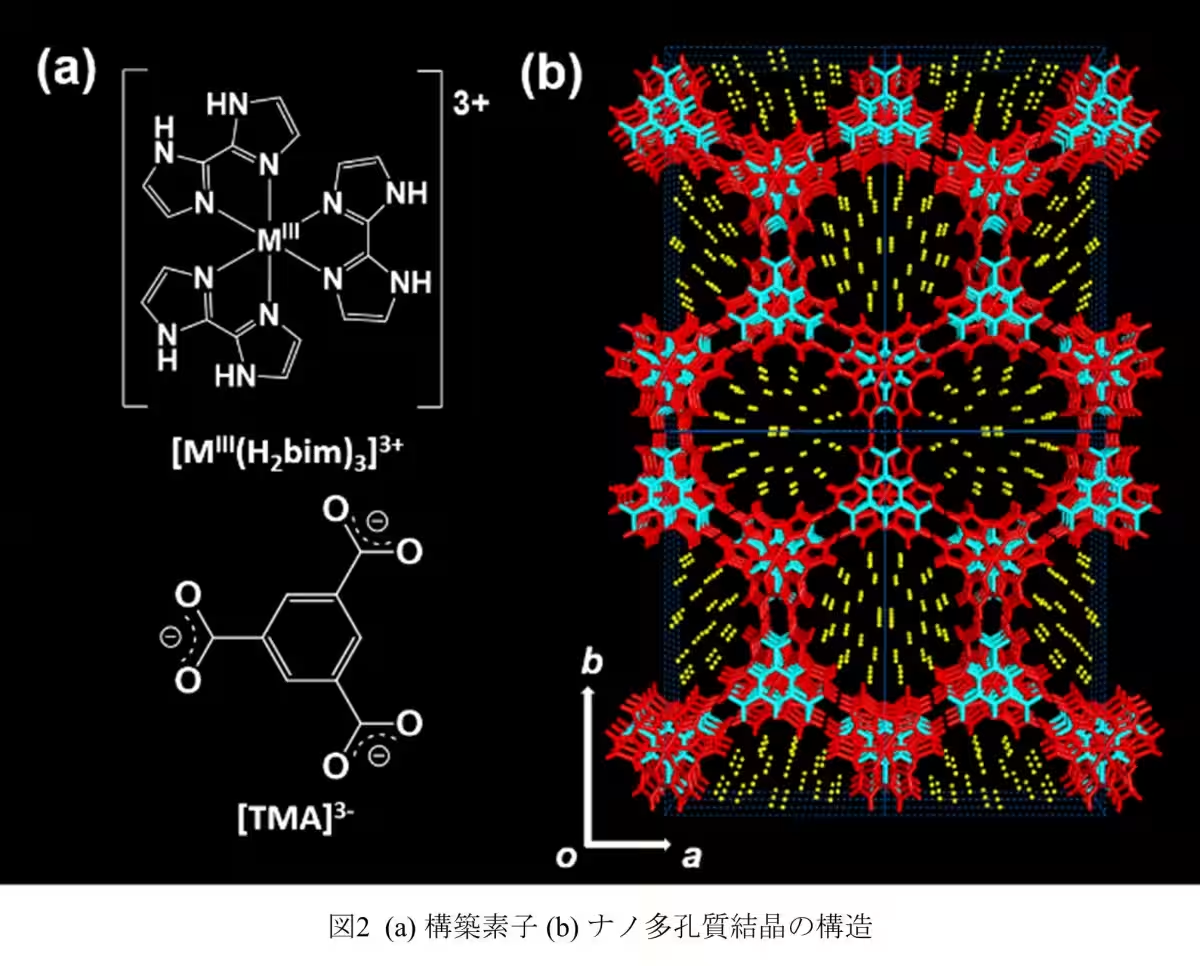
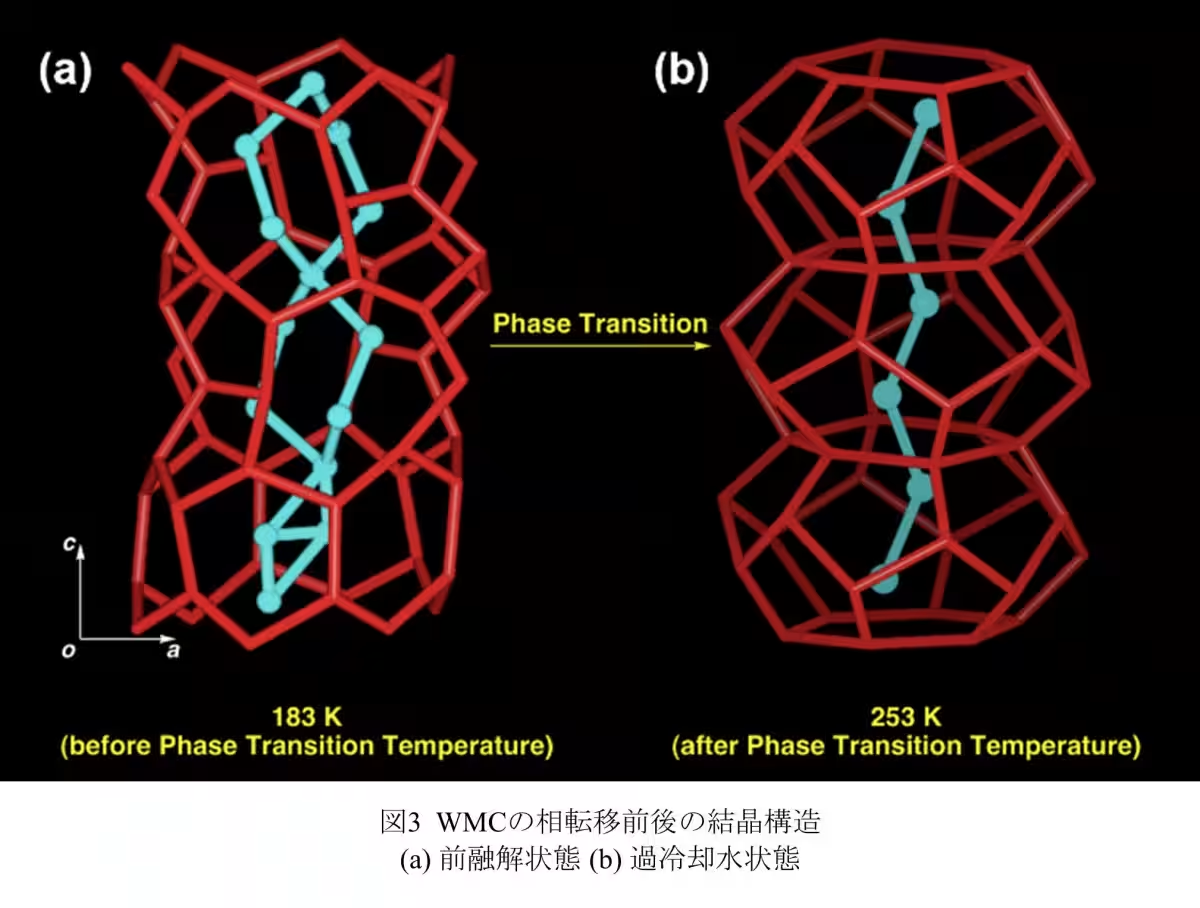
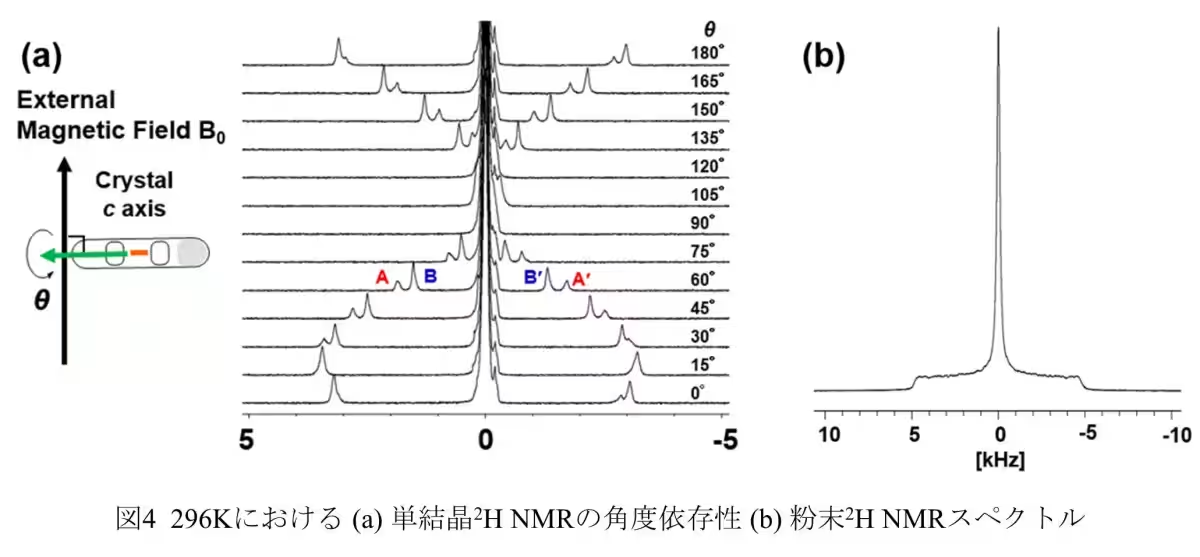
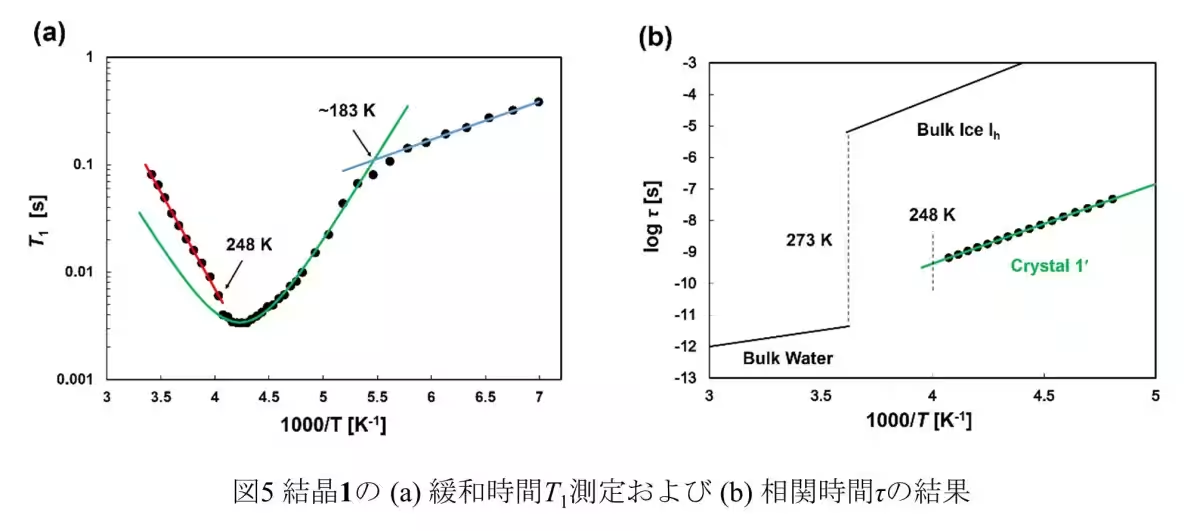
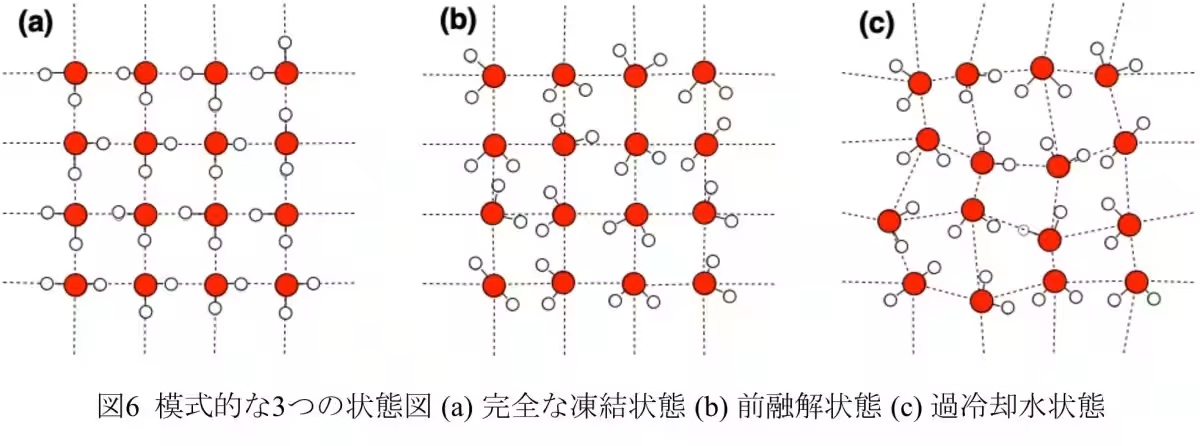
The team synthesized molecular nanocrystals with one-dimensional pores less than 1.6 nm wide. This allowed them to employ static solid 2H NMR to investigate the hierarchical structure of the D2O clusters. Their findings demonstrated the presence of a new pre-melting state, a critical phase preceding melting that changes the dynamics of water molecules significantly.
Key Findings
The study found that when water clusters are confined in this manner, they do not just freeze but can exist in a pre-melting state before reaching typical melting conditions. In this state, some D2O molecules maintain a fixed position, resembling solid ice, while others begin to move more freely, exhibiting liquid-like motion. This duality indicates a transitional phase that challenges conventional understandings of water behavior in such environments.
Measurements of the relaxation time T1 of solid 2H NMR revealed that while the oxygen atoms in the D2O clusters remain in fixed positions akin to ice, the deuterium atoms display dynamic movement characteristic of liquid water. This finding supports the theory that confined water behaves distinctly from bulk water—a critical factor for understanding biological membranes, ion diffusion, and energy storage applications.
Implications for Future Research
Professor Tadokoro emphasized the importance of these findings, asserting that they pave the way for deeper insight into the structural science of water and its clusters. The discovery of the pre-melting state suggests that water in confined spaces does not merely mimic its bulk behavior but interacts with surfaces in ways that potentially influence biological processes and material science.
As noted, the implications of this research extend beyond pure academic interest. The ability to manipulate water's state within nanocrystalline structures could lead to advancements in energy storage technologies, particularly in creating new forms of inexpensive materials utilizing hydrogen and methane gas storage.
Additionally, understanding the dynamic behavior of water at solid-liquid interfaces opens new pathways for exploring interface science and could provide insights into fundamental biological processes concerning water movement across cell membranes.
Conclusion
The synthesis of nanocrystalline structures and the discovery of the novel pre-melting state showcases the versatility and complexity of water behavior. This study, published in the Journal of the American Chemical Society, signifies a remarkable leap in material science and its interrelationship with biological processes, presenting a refreshed outlook on the science of water, a substance that remains central to life and industrial applications alike.
Further research inspired by these findings may redefine our comprehension of water and its roles in different environments, ultimately influencing both scientific inquiry and technological innovation.
Topics Other)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.