
Experimental Evidence Shows Molecular Anions Outperform Lithium Ions in Battery Applications
Introduction to Advances in Battery Technology
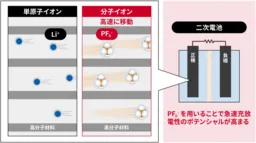
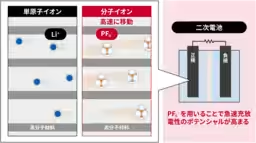
In a groundbreaking study, researchers at the National Institute of Advanced Industrial Science and Technology (AIST), along with Osaka Metropolitan University's College of Technology and Ehime University, have demonstrated that molecular anions, specifically hexafluorophosphate (PF6-), exhibit superior mobility compared to lithium ions (Li+) within the electrodes of secondary batteries. This discovery enhances our understanding of battery performance, particularly regarding fast charging and discharging capabilities.
Key Findings
Researchers employed specialized polymer materials in their experiments, allowing for a direct comparison of the movement of anions and cations under identical conditions. The results confirm that PF6- travels more rapidly than Li+ in solid-state electrodes, indicating the potential of molecular ion batteries to outperform traditional lithium-ion technologies.
Background on Lithium-Ion Batteries
Currently, lithium-ion batteries dominate the rechargeable battery market due to their high energy density. However, the mobility of Li+, which serves as the charge carrier, is often hindered by strong interactions with solvent molecules in electrolytes. This limitation affects charging times and overall efficiency, necessitating further advancements in battery technology.
Exploring Molecular Ion Batteries
The concept of molecular ion batteries, introduced by AIST in earlier research, focuses on employing molecular-sized anions as charge carriers. Unlike smaller monatomic ions such as Li+, molecular ions have a larger ionic radius and interact less intensely with solvents. This inherent difference provides them with a speed advantage that researchers have previously noted in liquid electrolytes; however, direct comparisons within solid electrodes had never been successfully conducted — until now.
Innovative Research Methodology
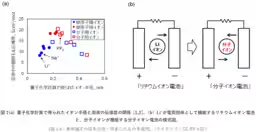
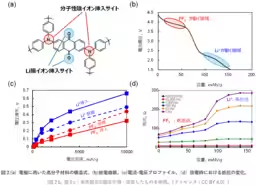
To address this challenge, the team utilized a novel polymer material designed to accommodate both cation and anion transport. This material integrates properties of quinone structures for Li+ and triphenylamine-derived structures for PF6-. This allowed researchers to examine the ionic mobility under the same experimental conditions, providing valuable insights into the relative speeds of these two types of ions in a solid-state setting.
Results and Implications
The findings show that PF6- has lower voltage loss and resistance during movement compared to Li+. Such characteristics imply that molecular ions can enhance the efficiency of charging and discharging processes within batteries. Moreover, this research opens new possibilities for designing secure batteries that mitigate risks associated with dendrite formation — a significant safety concern in lithium-ion technology.
Future Directions
As molecular ion batteries are still in the experimental phase, researchers aim to develop their electrode materials to enhance energy density, operational voltage, and safety further.
Publication Information
This research will be published in the journal ChemSusChem on July 25, 2025, under the title "Molecular Anions Move Faster than Lithium Ions as Charge Carriers in the Organic Battery Electrodes: Insights from 2,6-Bis(diphenylamino)anthraquinone." The authors include Hikaru Sano, Aya Yoshimura, Masaki Sawada, Tatsuo Noda, Yohji Misaki, and Masaru Yao, highlighting the collaborative nature of this groundbreaking work.
Conclusion
The demonstration of faster ionic movement of hexafluorophosphate over lithium ions marks a significant step in battery research. As the technology progresses, the potential for molecular ion batteries to offer safer, faster, and more efficient energy storage solutions looks promising and is expected to play a crucial role in the future of secondary battery development.
Topics Consumer Products & Retail)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.