
Kenai Therapeutics Secures $8 Million Grant to Enhance Parkinson's Disease Therapy Development
Kenai Therapeutics Secures an $8 Million Grant for Parkinson's Disease Treatment
Kenai Therapeutics, a biotechnology company at the forefront of developing next-generation therapies for neurological disorders, has recently received significant funding from the California Institute for Regenerative Medicine (CIRM). The $8 million grant is aimed at propelling the clinical development of their lead product, RNDP-001, a promising treatment for idiopathic Parkinson's disease.
Background on Kenai Therapeutics
Founded in 2022 and based in San Diego, California, Kenai Therapeutics centers its research and development on innovative solutions for brain-related diseases using its proprietary platform based on induced pluripotent stem cells (iPSCs). This platform has garnered recognition for its groundbreaking potential in regenerative medicine. The company is committed to developing therapies that not only alleviate symptoms but aim to restore lost neuro-functionality, offering a new avenue of hope for patients suffering from Parkinson’s disease.
RNDP-001: A Revolutionary Approach
RNDP-001 distinguishes itself as an allogeneic neuron replacement therapy that targets the underlying cause of Parkinson's disease—loss of dopamine-producing neurons. Current treatment approaches primarily manage symptoms rather than address the disease itself, leading to a significant unmet medical need within the Parkinson's community. By aiming to restore motor functions through neuron replacement, RNDP-001 represents a paradigm shift in treatment methodology.
Howard J. Federoff, M.D., Ph.D., Co-Founder and Chief Medical Officer of Kenai Therapeutics, emphasized that RNDP-001 follows a fundamentally different perspective on Parkinson's disease. It focuses on regeneration rather than mere management of symptoms, highlighting the potential for significant improvements in patient quality of life.
Clinical Development and Fast Track Designation
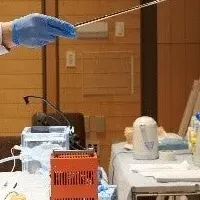
The funding from CIRM will be instrumental in advancing RNDP-001 through various critical stages of clinical trials. The therapy is currently assessed in a Phase 1 open-label study titled REPLACE™, which aims to evaluate its safety and efficacy in adult patients with moderate to severe Parkinson's disease. The trial also includes brain imaging studies to track the engraftment of the replacement neurons and assess any improvements in brain network function.
Recognizing the urgency for effective treatments, the U.S. Food and Drug Administration (FDA) has granted RNDP-001 the Fast Track designation. This status facilitates the development and review of the therapy, allowing for expedited approval processes due to the pressing need for new solutions.
The Impact of Parkinson's Disease
Parkinson's disease is a debilitating and progressive neurodegenerative condition. Over 10 million individuals worldwide contend with its impact, characterized by symptoms such as tremors, rigidity, and motor function decline alongside other issues like cognitive impairment and mood disorders. Current treatments primarily focus on alleviating symptoms rather than modifying the disease course. The substantial burden that Parkinson's imposes on individuals and their families underscores the need for innovative therapies that can enhance the management of the disease and improve life quality.
What CIRM's Support Means
CIRM's funding is aligned with its mission to accelerate stem cell and gene therapy advancements, aimed at addressing the medical community’s unmet needs. Their CLIN2 program specifically supports the translation of regenerative therapies into practical applications, fostering the creation of accessible treatments for patients.
As Kenai Therapeutics steers forward with the support of CIRM, both patients and advocates for regenerative medicine remain hopeful that RNDP-001 may unlock new opportunities in the treatment landscape of Parkinson's disease.
In conclusion, the journey of RNDP-001 signifies a beacon of hope for countless individuals facing the challenges of Parkinson's disease. As clinical trials progress, the anticipation surrounding its potential success continues to grow, promising to change the narrative surrounding neurological treatments.
Topics Health)








【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.