

Exploring Regenerative Medicine Growth in Asia: Market Forecasts and Regulations
The Rise of Regenerative Medicine and Cell Therapy in Asia
In recent years, the field of regenerative medicine and cell therapy has witnessed remarkable growth across Asia. According to a recent survey conducted by Seed Planning Inc., the market size for cell therapy and regenerative medicine is expected to reach approximately ¥1.47 trillion (about $13.4 billion) by 2025. This analysis highlights the significant growth trajectory of this market, alongside the ongoing development of relevant regulations and guidelines across various countries in the region.
Background and Purpose of the Study
The growing acceptance and integration of regenerative medicine in countries such as China, South Korea, and India have resulted in double-digit growth rates. Taiwan, Singapore, and Indonesia have also established regulatory frameworks for regenerative medicine, while countries like Korea and Thailand are in the process of developing similar regulations. The anticipation of future guidelines has drawn increased attention from industry stakeholders and regulators alike.
Moreover, the contract processing and cultivation business in cell therapy, particularly in Japan and China, is becoming increasingly active. There is an expectation that this trend will extend to other regions, fostering new opportunities for contract manufacturing and growth in the cell processing sector.
Given the promising developments, this comprehensive study aimed to analyze and understand the current landscape of regenerative medicine, its market dynamics, and the prevailing regulatory issues within Asia.
Key Insights from the Analysis
- - The study examines the market size trends and regulatory developments in the field of regenerative medicine and cell therapy across 11 Asian regions.
- - It differentiates between approved treatments under insurance and those offered without approval, providing a clearer understanding of the market.
- - The analysis also sheds light on the burgeoning contract business sector accompanying the commercialization of regenerative medicine.
- - Furthermore, it discusses the current legal frameworks and guidelines for regenerative medicine and cell culture facilities, as well as the medical insurance scenarios in each participating country.
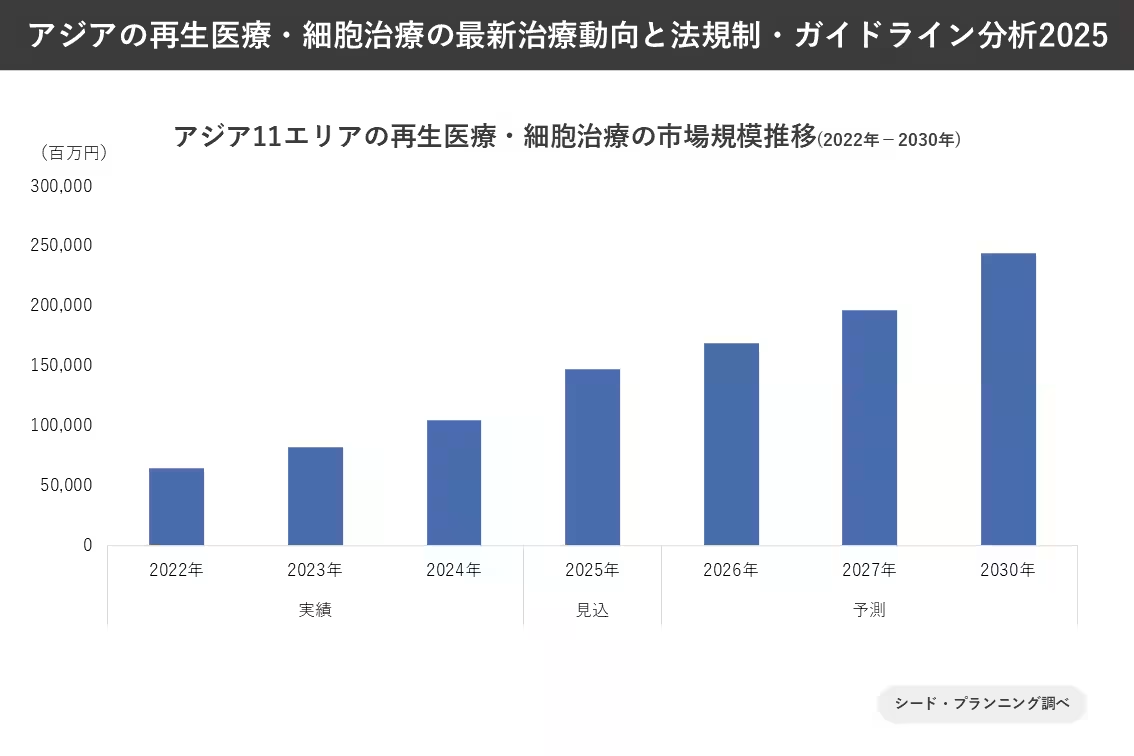
Market Growth Projections
Asian markets are witnessing a robust increase with revenue projections expecting to escalate over the following years. As of 2024, the market for self-funded regenerative therapies in these regions was estimated to be around ¥1 trillion. However, by 2025, it is forecasted to grow to approximately ¥1.47 trillion, with projections reaching as high as ¥2.44 trillion by 2030. This steady market expansion indicates a burgeoning interest and investment in regenerative medical technologies.
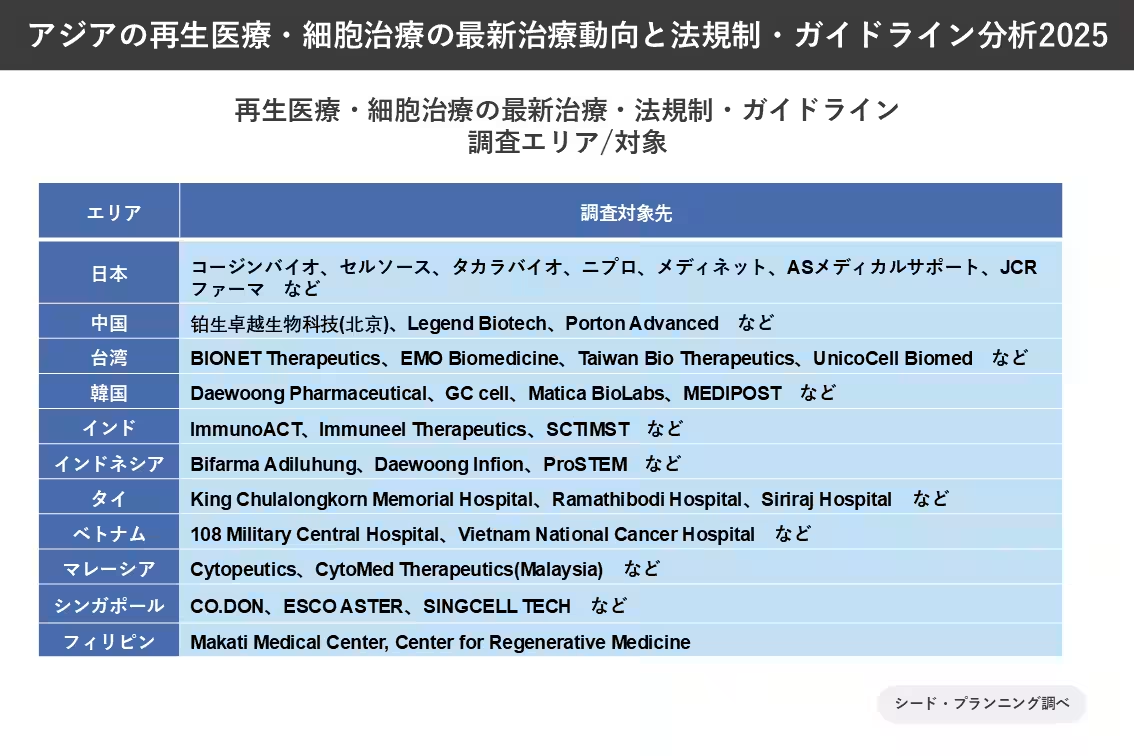
The breakdown of the study regions includes:
- - Japan
- - China
- - Taiwan
- - South Korea
- - India
- - Indonesia
- - Thailand
- - Vietnam
- - Malaysia
- - Singapore
- - Philippines
The analysis encompasses over 70 companies involved in regenerative medicine, including notable contributors such as Coijin Bio, Cellsource, Takara Bio, Nipro, Medinet, AS Medical Support, and JCR Pharma, among others.
Research Methodology
The research methodology employed a comprehensive approach including interviews and internet surveys to gather valuable data and insights on the following aspects:
- - The regenerative medicine and cell therapy market
- - Applicable regulations and guidelines in regenerative medicine
- - Healthcare insurance frameworks across the region
- - Contract processing and cultivation service market dynamics
Conducted from August to October 2025, the study aimed to provide a clear and detailed representation of the current and future landscape of regenerative medicine in Asia.
For more information about this study, visit Seed Planning's official site.
Contact for Inquiries
For inquiries regarding the data and information, please reach out to the Public Relations Department via email at [email protected].

Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.