

Unexpected Commonalities Among Different Amorphous Materials Unveiled by Researchers
Unveiling the Surprising Similarities Among Amorphous Materials
A Breakthrough in Material Science
The recent research conducted by a team led by Professor Akihiko Hirata at Waseda University and Researcher Kengo Nishio from the National Institute of Advanced Industrial Science and Technology (AIST) has presented a groundbreaking revelation in the field of material science. Traditionally, metal oxides formed through ionic bonds were considered structurally distinct from alloys formed through metallic bonds. However, this study has experimentally confirmed that both types can possess strikingly similar atomic arrangements when in an amorphous state.
Key Findings
The researchers revealed that in amorphous materials like hafnium oxide (HfO2), the arrangement of atoms can resemble that of metallic alloys, showcasing a densely packed atomic structure comparable to that of tightly clustered pegs in a pachinko machine. This unexpected discovery challenges long-held conventions about the structures of materials, especially regarding their atomic configurations.
The research primarily focused on HfO2, a critical material known for its use in electronic devices. Through advanced observation techniques, they demonstrated that the atomic arrangements of Hf atoms within the amorphous oxide are indeed randomly packed, akin to the structures observed in certain amorphous alloys such as zirconium-platinum (Zr80Pt20).
The Scientific Process
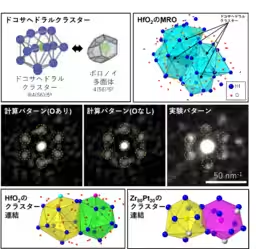
Utilizing innovative atomic-level observation technologies and analytical methods, the team confirmed the theoretical predictions about the similarities in atomic arrangement. Specifically, they employed Angstrom Beam Electron Diffraction (ABED) and Polyhedral Code methods to scrutinize the local structures of amorphous materials, which allowed them to observe the unique packing arrangements of metal ions within the glass-like structures.
Figure 1 from their findings illustrates the unique local atomic arrangements formed by dodecahedral clusters, which serve as the building blocks in both HfO2 and Zr80Pt20, leading to the establishment of medium-range order (MRO) structures. This mutual architectural feature affords a new insight into the understanding of complex amorphous structures, now simplified to a common framework.
Implications for Future Material Development
The ramifications of this discovery are multifaceted. With a unified understanding of the structural similarities between amorphous metal oxides and amorphous alloys, future research can exploit the newfound correlations for innovative material developments. For instance, if methods are developed to mitigate dodecahedral clusters in amorphous alloys, it may simplify the amorphization process of metal oxides, opening doors to novel applications and efficiencies in materials science.
Furthermore, the insight gained from this study suggests that similar structures could exist across various amorphous materials. This could lead to an enhanced capacity in the material innovation landscape, including advancements in semiconductor materials where HfO2 is often used.
Conclusion
In a world where science often seeks to simplify the intricate web of materials into understandable principles, this discovery helps illuminate the underlying connections between seemingly disparate constellations of atomic arrangements. The work of Hirata and Nishio not only bridges gaps in scientific understanding but also sets the stage for future technological advancements in fields that utilize amorphous materials. As ongoing research continues to unfurl the layers of complex material structures, society may soon benefit from revolutionary innovations built on these foundational understandings.
Future Prospects
While significant strides have been made, questions remain unresolved regarding the atomic sequence of oxygen in the amorphous structure and whether similar atomic arrangements can be observed in other metal oxides or complex amorphous compounds. The potential impact of the dodecahedral cluster structure on the performance of amorphous materials and their production processes requires further investigation. The research team aims to expand their focus to encompass different amorphous materials to validate the existence of these shared structures, paving the way for new breakthroughs.
Quotes from Researchers
Professor Hirata expressed enthusiasm over their success in observing hafnium's atomic arrangement. He mentioned, "This success reinforces the utility of Angstrom Beam Electron Diffraction in analyzing amorphous structures."
Kengo Nishio remarked on the challenges faced during the theoretical predictions but noted the feasibility that has emerged through experimental verification. "As barriers to examination are lowered, opportunities for theoretical researchers to conduct more applicable predictions grow."
Additional Information
For further reading, details of this research are documented in the forthcoming article in Communications Materials:
- - Author: Akihiko Hirata, Waseda University; Kengo Nishio, AIST; Masayuki Okukawa, Osaka University; Ryusuke Nakamura, Shiga University
- - Expected Publication Date: July 30, 2025
- - DOI: https://doi.org/10.1038/s43246-025-00894-0
Keywords: Amorphous structures, Random close packing, Amorphous metal oxides, Amorphous alloys, Dodecahedral clusters, Angstrom beam electron diffraction, Polyhedral code.

Topics Consumer Products & Retail)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.