
Revolutionary Fertility Device “kegg” Set for Japanese Launch in 2025
Introduction to kegg
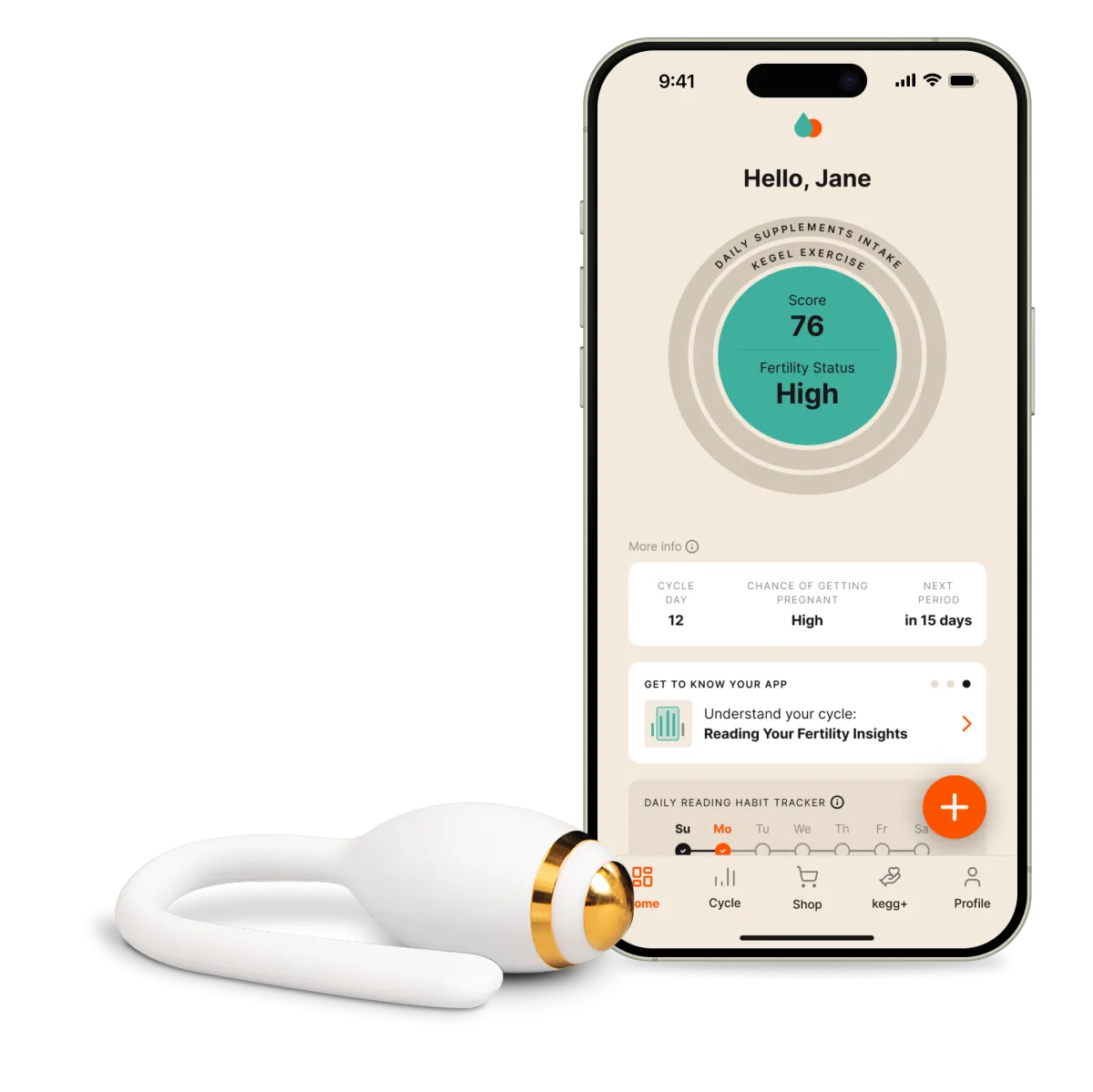
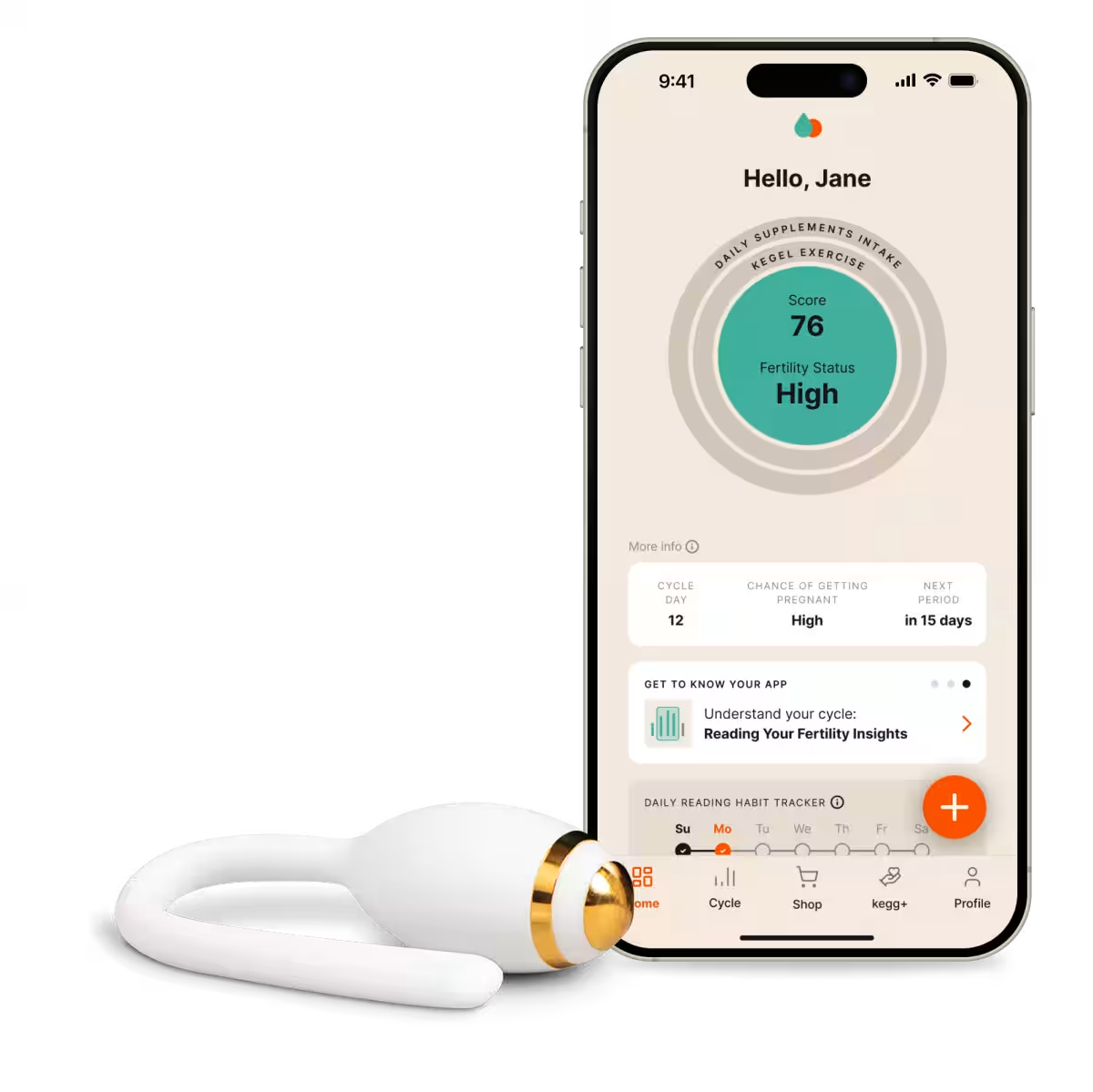
The acclaimed fertility-tracking device, kegg, is making waves as it sets its sights on the Japanese market for general sale in the summer of 2025. Developed by the American company Lady Technologies and introduced in collaboration with fermata, a Tokyo-based firm, this state-of-the-art device promises to revolutionize how couples approach fertility.
Key Features of kegg
kegg functions as both a fertility tracker and a pelvic floor trainer, leveraging advanced sensing technology to analyze changes in cervical mucus. This analysis allows users to identify their ovulation period and fertile window with precision. By utilizing Electrochemical Impedance Spectroscopy (EIS), kegg only requires a 90-second insertion to collect and transmit cervical data, which users can then monitor in real time through a dedicated app. Officially recognized as a Class I Medical Device in Japan in 2024, kegg has already supported the fertility journeys of over 50,000 women and couples, primarily in the United States.
Background and Development
The partnership between fermata and Lady Technologies began in 2019, focusing on tailoring kegg for the Japanese market. Following rigorous clinical validations, product registrations, and the development of a localized application, the groundwork has been laid for a successful launch. Furthermore, in anticipation of this launch, Lady Technologies successfully secured $6.5 million in Series A funding in 2024.
Significance for the Japanese Market
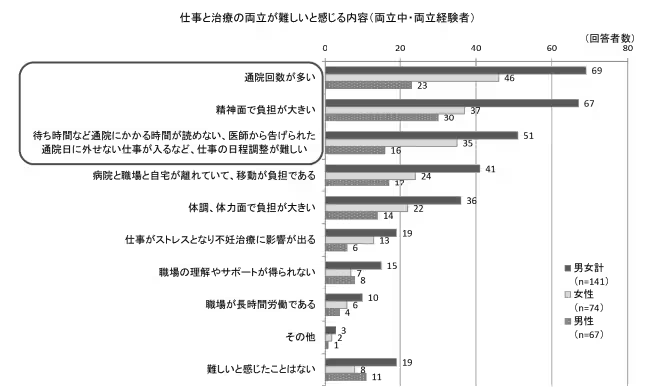
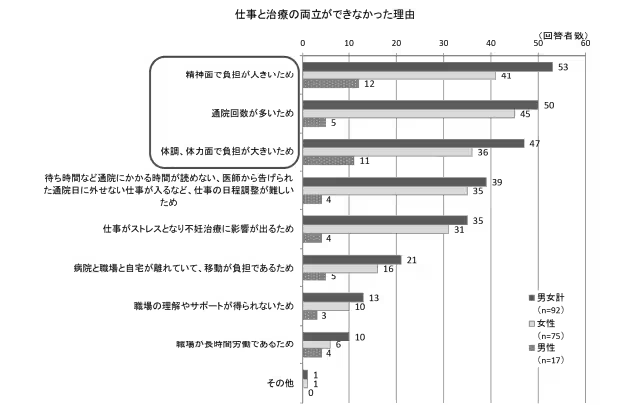
In Japan, infertility is a prevalent concern, with one in three couples expressing unease regarding their fertility. Traditional methods, such as basal body temperature tracking or urine tests, have proven to be inconsistent and often burdensome, emphasizing the need for innovations such as kegg. By shifting the responsibility for fertility monitoring into the hands of individuals, kegg aims to alleviate the physical, emotional, and financial strains many couples face when trying to conceive.
Frequent visits to medical facilities purely for ovulation tracking can be inefficient, and kegg addresses this gap by offering an at-home solution. The device allows users to integrate fertility monitoring into their daily routines, thereby alleviating stress for both users and healthcare practitioners. The ease and affordability of such self-monitoring can promote earlier awareness, enhancing timely interventions and combatting the trend of delayed fertility treatments.
Journey Towards Launch
- - 2018: Preliminary discussions between fermata and Lady Technologies commence.
- - 2019: fermata is officially established in Japan.
- - 2020: fermata invests in Lady Technologies to support product development.
- - 2021: kegg launches in the U.S.; a clinical study begins in collaboration with the University of Tokyo.
- - 2022: Expansion of kegg sales in the U.S. market.
- - 2023: Japan's Ministry of Health recognizes kegg as a new medical device category.
- - 2024: kegg is registered as a Class I Medical Device in Japan, and the device is showcased at the Japan Society of Obstetrics and Gynaecology's annual meeting.
Statements from Leadership
Amina Sugimoto, CEO of fermata, expressed her excitement about introducing kegg to the Japanese population after five years of dedicated preparation.






Topics Other)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.