
REGAL CORE's Regular Survey: Analyzing Potential Violations of Pharmaceutical Laws and Advertisement Regulations
Regular Survey by REGAL CORE
In an ongoing effort to ensure compliance with pharmaceutical laws and advertising regulations, REGAL CORE Co., Ltd., based in Shibuya, Tokyo, is conducting regular surveys to identify potentially misleading advertisement expressions. This article summarizes the findings of the most recent survey conducted from August to September 2025, observing that illegal advertisement expressions remain prevalent in article landing pages (LPs) across various web media.
Survey Overview
The survey aimed to scrutinize article LPs that appeared in randomly selected web media, focusing specifically on advertisements displayed through recommended widgets. The analysis involved monitoring these ads bi-weekly, assessing their claims based on the pharmaceutical laws and advertising regulations to protect consumers from misleading propositions.
Findings
Over the surveyed period, many of the identified article LPs contained advertisement expressions that might violate the Pharmaceutical Affairs Law and the Act against Unjustifiable Premiums and Misleading Advertisements. Here are some example expressions that have raised concerns:
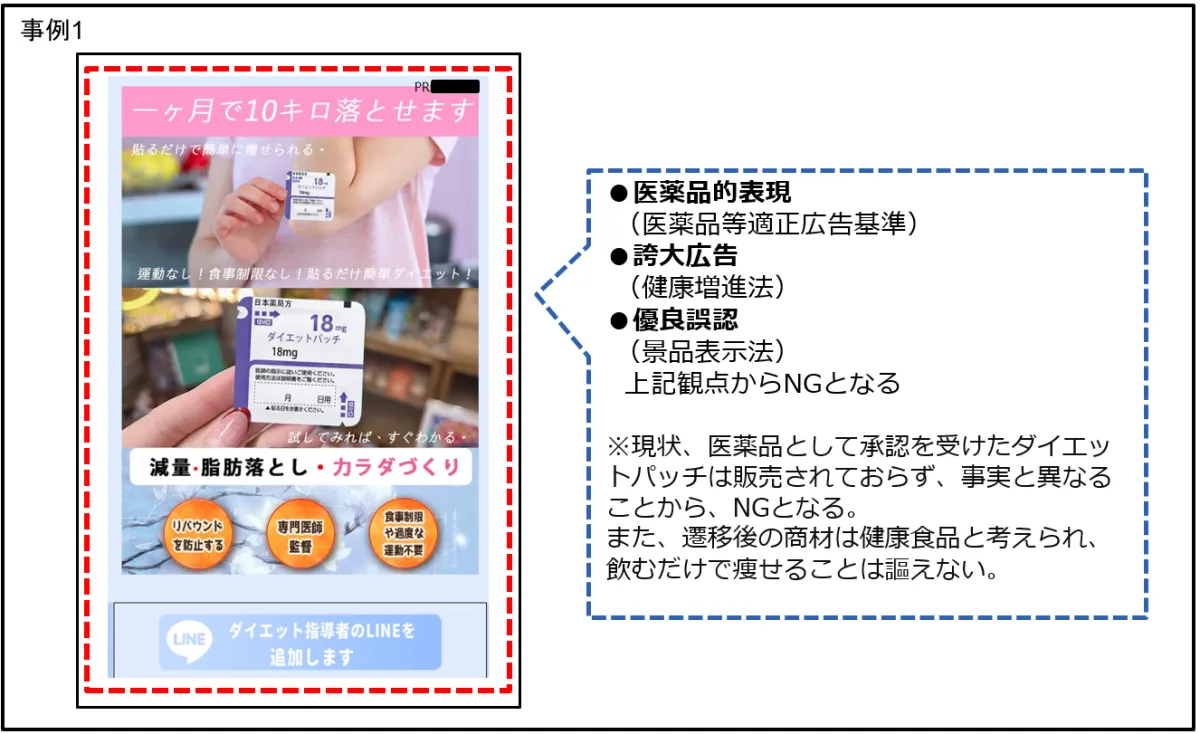
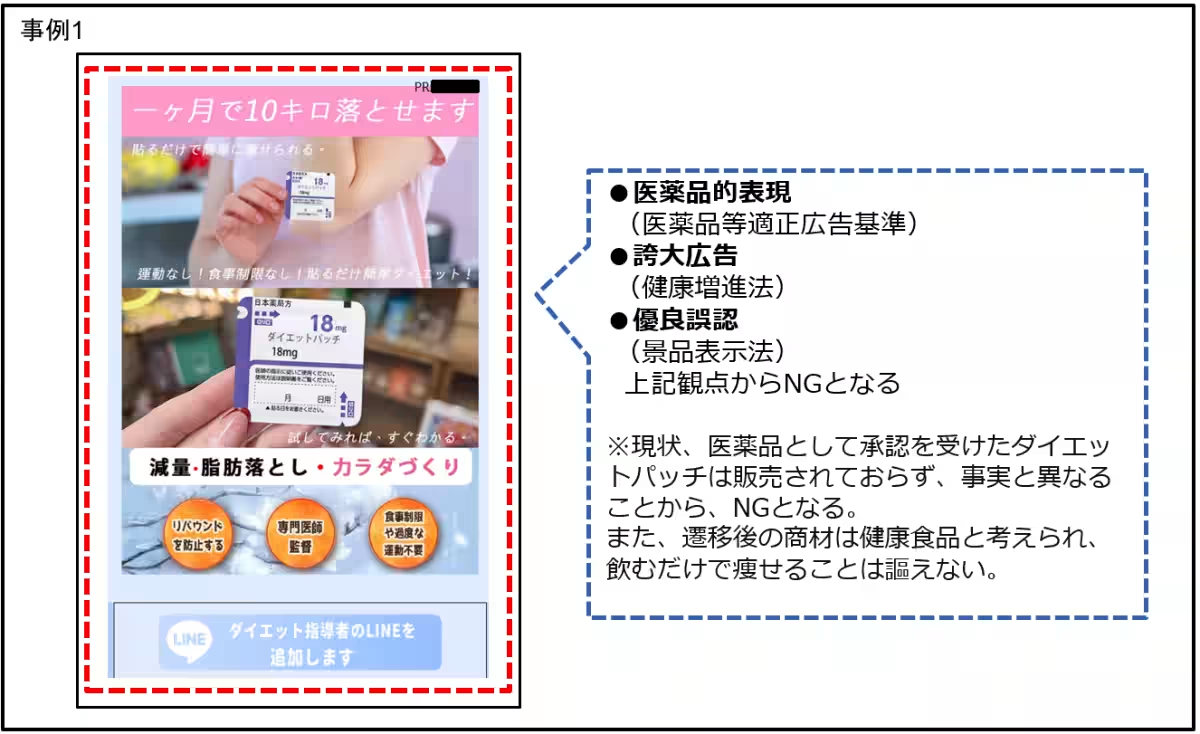
1. Health Supplements: Many products were found claiming effects akin to pharmaceuticals, making misleading assertions of their benefits. For instance:
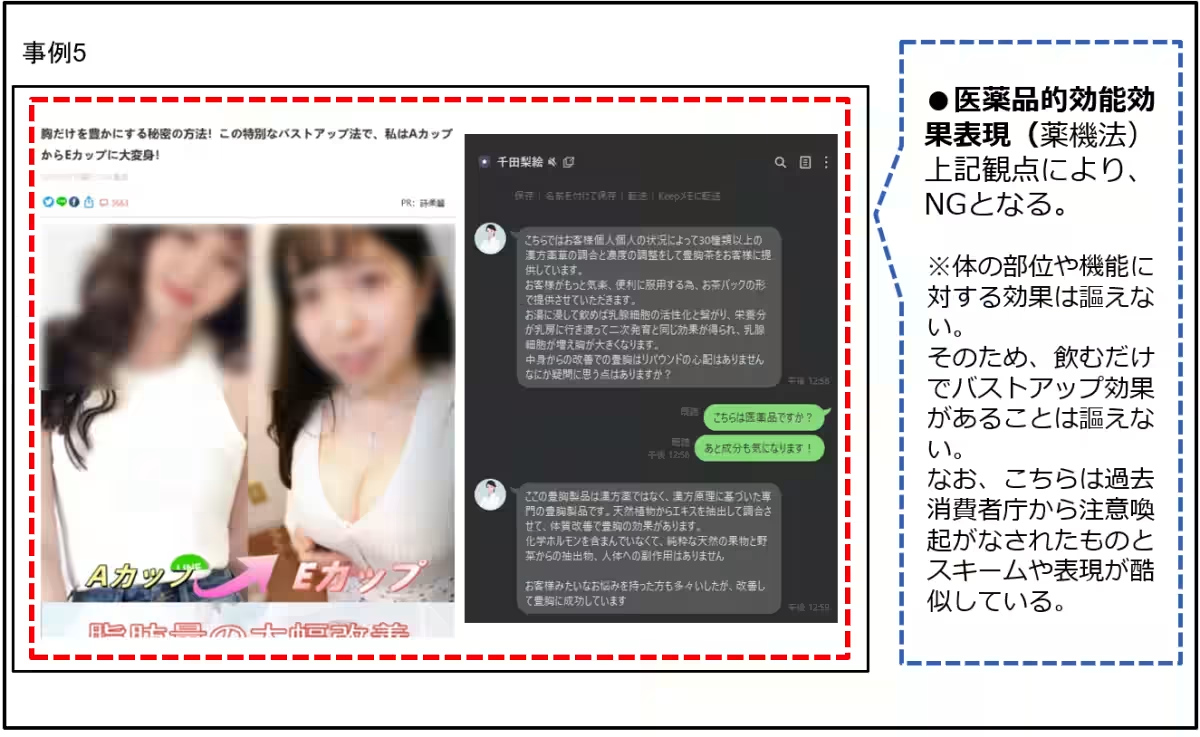
- Statements like “hormone support” implying breast enhancement or weight loss by simply taking a product contradict medical standards.
- Claims of detoxifying effects that suggest immediate improvement in skin quality or eliminating bad breath through ingestion, which border on fraudulent.
2. Functional Foods: Claims exceeding verified health benefits led to misleading advertisement. Examples include:
- Promoting products as capable of improving eyesight or metabolic rates without adequate scientific backing.
- Suggesting easy weight loss without diet or exercise, which is grossly misleading.
3. Cosmetics: Products in this category often violate standards with exaggerated effects on skin and hair conditions. Instances include:
- Advertising hair regrowth or skin rejuvenation that’s unverifiable or exceeds permissible advertising claims, such as representing a product as a permanent hair removal solution.
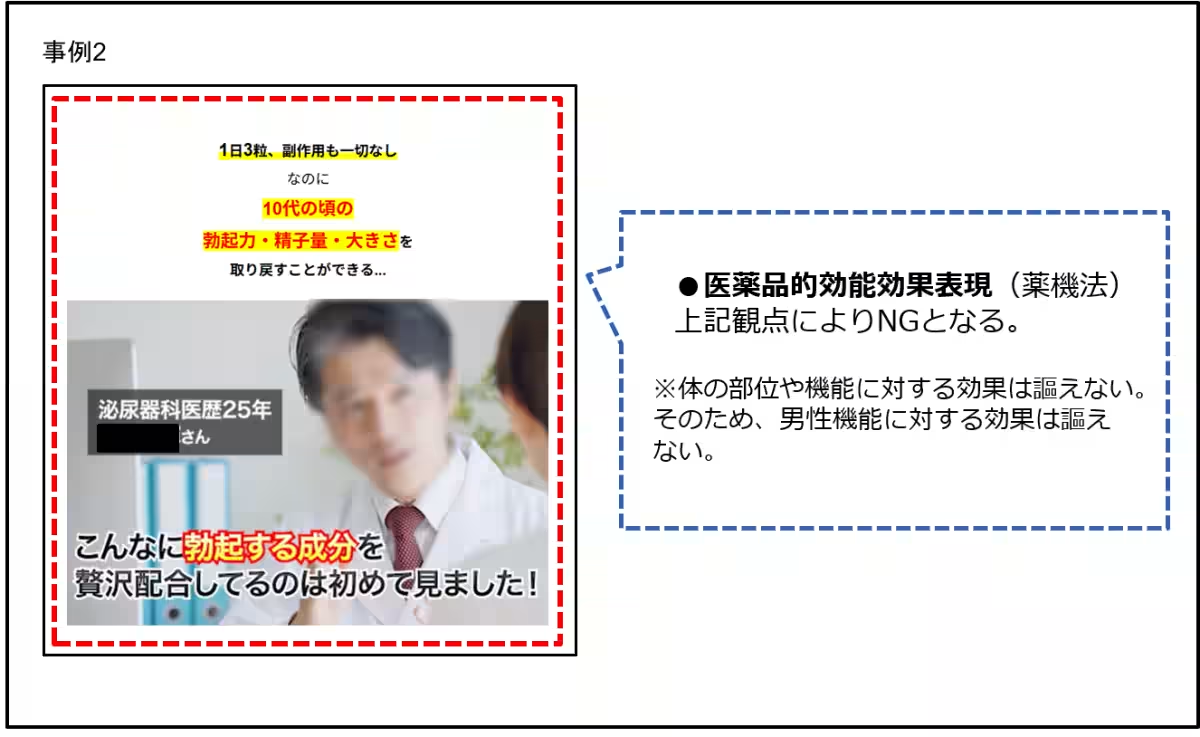
4. Pharmaceuticals: Some pharmaceutical advertisements incorrectly suggest they can cure or treat conditions, presenting misleading narratives about efficacy akin to treatments for serious diseases.
5. Expressions that Guarantee Results: A notable trend is the use of phrases promising guaranteed results, creating false confidence in the product’s effectiveness, which may violate consumer rights.
In reviewing the ongoing compliance of various advertisements, REGAL CORE has identified many businesses that have already corrected their problematic expressions following the previous surveys.
Conclusion
The survey concludes by emphasizing the need for continuous monitoring and updates regarding advertisement expressions that risk infringing on consumer protection laws. REGAL CORE has been diligently refining its investigation methods and interpretations since August 2022, committing to ongoing research and reporting on compliant advertising practices within the industry.
Company Overview
Founded in 2021, REGAL CORE specializes in developing and providing advertising expression check tools, offering consulting for advertisement audits and regulation design. With a capital of 38.49 million yen and located in Shibuya, Tokyo, REGAL CORE is dedicated to enhancing the integrity of advertising across Japan.
For further information, visit REGAL CORE.



Topics Business Technology)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.