

NorLevo® Launches Japan's First OTC Emergency Contraceptive Pill
Introduction
Japan is set to witness a significant shift in reproductive health options with the introduction of NorLevo®, the country’s first over-the-counter (OTC) emergency contraceptive pill. Launched by Daiichi Sankyo Healthcare Co., Ltd., this product will be available from February 2, 2026. The introduction aims to enhance access to emergency contraception, ensuring that women have the means to prevent unintended pregnancies without the need for prescriptions.
Background
NorLevo® has been a trusted name in emergency contraception since 2011, when it was first introduced as a prescription-only medication. The new OTC version contains the same hormone—levonorgestrel—used in the original formulation, which is recognized globally as an essential medication by the World Health Organization (WHO). This brand is designed to assist women in managing unforeseen circumstances around contraception, providing a timely solution.
Key Features of NorLevo®
- - Target Audience: NorLevo® serves as a vital medication for women facing the risk of unintended pregnancy due to contraceptive failure or lack of access to other options.
- - Formulation: The product features levonorgestrel, a synthetic hormone that works primarily by preventing ovulation, inhibiting fertilization, and blocking the implantation of a fertilized egg.
- - Efficacy: When taken within 72 hours after unprotected sexual intercourse, the pill boasts an effectiveness rate of approximately 81%. The sooner it is consumed, the more effective it is at preventing pregnancy.
Purchase and Accessibility
For women seeking to purchase NorLevo®, specific protocols must be followed:
- - Consultation Requirements: Only pharmacists who have undergone specialized training in emergency contraception are authorized to facilitate the sale of NorLevo®. This is to ensure that women receive the appropriate guidance before use.
- - Sales Restrictions: The medication can only be sold directly to the individual intending to consume it, without the need for parental or partner consent, and there are no age restrictions.
- - Administration in Store: It is imperative that NorLevo® be taken under the supervision of a pharmacist at the point of sale, rather than being taken home for later consumption.
- - Eligible Cases: If the sexual encounter occurred more than 72 hours prior or if certain health warnings apply, the sale may be prohibited.
Socioeconomic Context
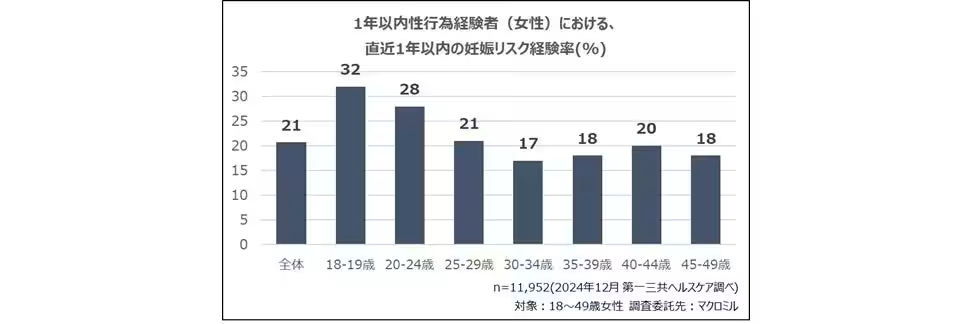
Recent studies indicate that a significant proportion of women aged 18 to 49 in Japan have experienced sexual activity within the past year, translating into millions of potentially vulnerable individuals facing the risks associated with unintended pregnancies. Current contraceptive methods frequently encounter failures, particularly with male condoms, which may have a failure rate of between 2% and 13%. This reality underscores the necessity of accessible emergency contraceptive options, particularly in light of Japan's rising abortion rates, especially among younger demographics.
Commitment to Education
Daiichi Sankyo Healthcare is committed to not only providing NorLevo® but also fostering a comprehensive understanding of reproductive health. As the OTC emergency contraceptive joins the market, the company plans to bolster awareness and education programs aimed at informing women of their options and rights regarding contraceptive care.
Conclusion
The launch of NorLevo® signifies an important milestone in Japan’s approach to reproductive health. By making emergency contraception readily available over the counter, Daiichi Sankyo Healthcare is taking a critical step towards empowering women and addressing serious health concerns around unintended pregnancies. As part of a broader commitment to health and well-being, the NorLevo® brand aims to educate and provide resources for women, fostering a choice-driven approach to reproductive health management.
For further information, visit the official NorLevo® brand site (opening mid-January 2026): NorLevo® Brand Site.


Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.