
Revolutionizing Chemical Production from Fermentation Gases at Low Temperatures
Revolutionizing Chemical Production from Fermentation Gases at Low Temperatures
The conversion of fermentation gases into chemical raw materials is receiving significant attention in the realm of sustainability. Researchers at Waseda University, in collaboration with Crasus Chemical Inc., have made a groundbreaking advancement in producing synthetic gas from biogas—specifically, a mix of methane and carbon dioxide—efficiently and at low temperatures. This remarkable technique emerges as a solution to some of the environmental challenges posed by these two potent greenhouse gases, which contribute significantly to global warming.
The Traditional High-Temperature Method
Previous methods of converting methane and carbon dioxide into chemical raw materials necessitated extreme temperatures, typically around 800 degrees Celsius. This process was not only energy-intensive but also led to significant carbon deposition, rendering it impractical for large-scale industrial applications. The high temperatures caused the formation of solid carbon, which negatively affected the catalyst's efficiency and longevity, ultimately hindering the continuous production of synthetic gas.
A New Approach
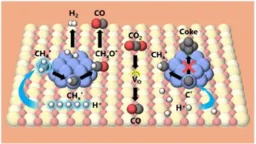
In this innovative research, Professor Yasushi Sekine and his team at Waseda University have developed a novel catalyst: a 1wt% Ru/La₂Ce₂O₇-supported catalyst. This catalyst allows the dry reforming of methane to be carried out at pressures high enough to prevent carbon buildup, while successfully operating at temperatures below 200 degrees Celsius. This is a significant departure from the standard methods, vastly improving both efficiency and output.
Through the utilization of unique ionic surface dynamics, the researchers were able to enhance catalyst performance at these unprecedented low temperatures. Their results indicate a high conversion rate of methane and carbon dioxide and a favorable hydrogen-to-carbon monoxide ratio, crucial for the subsequent use of synthetic gas in chemical manufacturing.
Environmental Impact
The technology represents a vital step towards the carbon-neutral era, capitalizing on the underutilized resource of biogas obtained from organic waste. By efficiently converting this biogas into chemical feedstocks on demand, the researchers pave the way for more versatile applications across various industries. Given the increasing emphasis on sustainable practices, this new methodology not only addresses waste management but also contributes to reducing greenhouse gas emissions by converting biogas—a resource often considered a liability—into valuable materials.
Future Directions
The collaboration between Waseda University and Crasus Chemical Inc. is set to continue, with ongoing research aimed at scaling up the process for industrial applications. The project marks a promising avenue for the advancement of sustainable chemical production, suggesting that further optimization could yield even greater efficiencies and capabilities.
In summary, the findings are set to be published on July 18, 2025, in ACS Catalysis, addressing a long-standing challenge in the field of chemical engineering. Their success in developing a stable and efficient means to derive chemical raw materials from biogas at low temperatures marks a significant milestone in the quest for sustainable industrial practices.
Conclusion
This pioneering work not only opens the door to new possibilities in chemical production but also highlights the importance of interdisciplinary approaches in tackling complex environmental challenges. As society continues to seek greener alternatives within industry, innovations like these play a crucial role in driving forward progress toward a sustainable future.
Topics Consumer Products & Retail)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.