

Sanso Pharmaceutical Releases PRISMA2020-Compliant Ingredient List for Functional Foods
Sanso Pharmaceutical Unveils Comprehensive Ingredient List
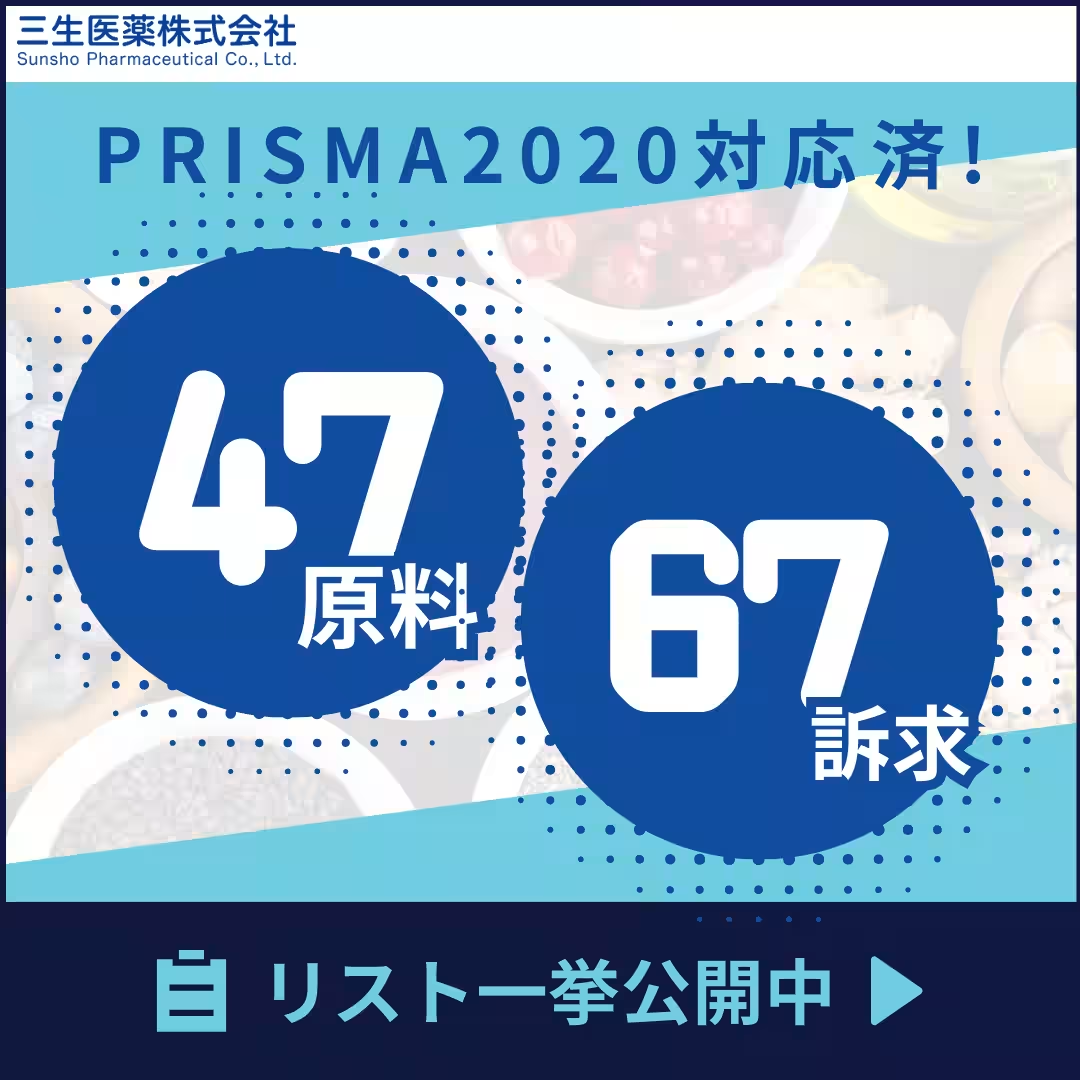
Sanso Pharmaceutical Co., Ltd., based in Fujiyama, Shizuoka, is revolutionizing the health supplement industry by releasing a detailed list of 47 ingredients and 67 health claims that comply with the PRISMA2020 guidelines. This list is part of the updated regulatory framework for functional foods set to take effect on April 1, 2025, which mandates compliance with systematic review principles.
Due to recent modifications in the functional food labeling system, the quality of compliance has become as critical as the compliance itself. The new regulations require systematic reviews that align with PRISMA2020 standards, intensifying the need for OEM partners who possess both expertise and a successful track record of accepted notifications.
A Step Forward in Product Development
By adapting to these changes, Sanso Pharmaceutical reinforces its commitment to not only meeting regulatory expectations but also leading the industry in product quality. With their updated list, manufacturers can easily identify compliant ingredients that have been documented and accepted under the new guidelines.
Some examples from the newly released PRISMA2020-compliant ingredients include:
- - Raphanus Extract: Health Claim - Improved sleep quality.
- Benefit: Reportedly enhances deep sleep and satisfaction upon waking.
- - GABA: Health Claim - Stress relief.
- Benefit: Known for its effectiveness in alleviating temporary mental stress and fatigue associated with work or study.
- - Astaxanthin (Hematococcus Pluvialis Extract): Health Claim - Eye health.
- Benefit: Aids in maintaining eye focus adjustments and alleviates eye strain from prolonged screen exposure.
These examples reflect just a portion of the diverse health claims supported by Sanso Pharmaceutical's ingredient lineup. In total, these ingredients assist with a variety of health issues ranging from cognitive function to digestive support and skin health.
Commitment to Quality and Speed
Sanso Pharmaceutical's Executive Officer, Mizue Sugiura, commented on the regulatory changes: “This amendment signifies not merely compliance but a shift towards prioritizing the quality of adaptation among OEM manufacturers. With our robust background in formulation development and application success, we aim to transform regulatory changes into opportunities for our partners.”
They emphasize the integration of comprehension, speed, and execution as key elements in fostering successful product development. Companies are encouraged to seize the chance to collaborate with Sanso for their OEM solutions, offering tailored support from the initial concept through product launch and regulatory submission.
Moving Beyond Compliance
Ultimately, Sanso Pharmaceutical presents these updates not as a mere regulatory necessity but as a pivotal starting point for delivering trusted products to consumers. As the industry prepares for additional changes in 2025, companies must position themselves with responsive and reliable OEM partners. Matched with proven effectiveness and agile support, Sanso aims to empower businesses in crafting a new generation of health products.
Contact Information
For product development consultations or insights into the compliance processes, interested parties can reach out to Sanso Pharmaceutical through their communication channels.
Media Contact:
Kenichi Fujisaku
Email: [email protected]
About Sanso Pharmaceutical Co., Ltd.
Founded in 1993, Sanso Pharmaceutical has been a pioneer in health food, pharmaceuticals, and general food products with a current aim to lead through innovation and quality assurance.
Location: 1468 Atsuhara, Fujiyama, Shizuoka
CEO: Akira Imamura
Capital: Approximately 1.23 billion yen
Revenue: 25 billion yen (FY ending March 2024)
Employees: 700 (as of January 2025)
Company Website


Topics Consumer Products & Retail)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.