
SmartSkin Introduces Innovative Seal Assurance Solution for Pharmaceuticals
SmartSkin Introduces Innovative Seal Assurance Solution
SmartSkin Technologies, a prominent leader in pharmaceutical manufacturing intelligence, has recently unveiled a groundbreaking solution known as SmartSkin Seal Assurance. This new technology empowers pharmaceutical manufacturers with real-time insights into the integrity of their container seals, enhancing the reliability and precision of the capping process. SmartSkin will showcase this state-of-the-art solution at the upcoming ISPE 4.0 event in Barcelona, Spain, where they will highlight how data analytics can significantly improve sealing quality in pharmaceutical production.
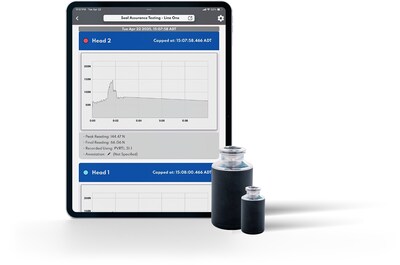
The primary functionality of SmartSkin Seal Assurance is to provide manufacturers with timely visibility into the performance of the sealing process. By meticulously measuring the forces involved during capping, this innovative solution generates a distinct digital signature for each capping event. This feature not only ensures that each sealing head is operating within specified parameters but also creates an auditable record of performance, thereby confirming the quality and consistency of the seal settings.
An important aspect of the Seal Assurance system is its compatibility with single or multiple head systems, which augments the traditional Residual Seal Force (RSF) testing. This capability guarantees that the sealing conditions observed during RSF are established from the onset, thus ensuring the overall integrity of the container closure.
Dr. Mihaela Simianu, a scientific advisor at SmartSkin Technologies, stated, "Despite the critical importance of sealing quality for sterile products, translating the mechanical adjustments of capping equipment into the necessary physical characteristics for container closure performance has been elusive—until now." She further emphasized that SmartSkin's Seal Assurance system opens up opportunities to proactively integrate container closure integrity assurance into the design of the aseptic process. This integration enables measurable, controllable, and reliable enhancements to equipment and process performance.
A recent implementation of the Seal Assurance technology with one of the world’s largest pharmaceutical manufacturers demonstrated its unique ability to detect specific inconsistencies per head and identify optimal capping parameters. This validation highlights Seal Assurance as a proactive control solution that simultaneously enhances quality and efficiency in pharmaceutical manufacturing.
The applications of Seal Assurance are broad, ranging from Factory Acceptance Testing (FAT/SAT) and line qualification to routine configuration verification and troubleshooting. SmartSkin Technologies is committed to providing manufacturing intelligence solutions that enhance product quality, improve patient safety, and mitigate costly production issues. By integrating advanced Digital Twins of Containers with real-time analytics, SmartSkin effectively assists manufacturers in identifying risks, preventing defects, and facilitating continuous improvement in the complex operations of filling, finishing, and packaging.
SmartSkin Technologies has gained the trust of global industry leaders, including Schott, Pfizer, and Sanofi, by delivering the precision and visibility needed to achieve defect-free production while upholding the highest standards of excellence in pharmaceutical manufacturing.
For more information about SmartSkin, visit www.smartskintech.com. For media inquiries, please contact Kumaran Thillainadarjah, Founder and Chief Technology Officer of SmartSkin Technologies, at [email protected] or call +1 (855) 210-9006.


Topics Consumer Products & Retail)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.