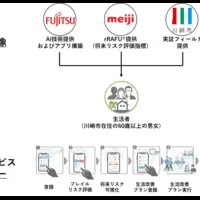
FDA Approves Phase 2 Trial for Promising New Drug AK3280 to Treat Pulmonary Fibrosis
FDA Approves Phase 2 Trial for AK3280
Shanghai Ark Biopharmaceutical Co., Ltd. (commonly referred to as ArkBio) has recently announced a significant development for its innovative drug AK3280. The U.S. Food and Drug Administration (FDA) has granted clearance for ArkBio to begin Phase 2 proof-of-concept clinical trials in the United States, aimed at treating idiopathic pulmonary fibrosis (IPF). This milestone brings renewed hope for patients suffering from this progressive and often fatal lung disease.
Understanding Idiopathic Pulmonary Fibrosis (IPF)
IpF is characterized by the gradual scarring of lung tissue, leading to respiratory failure and overall decline in lung function. The median survival rate post-diagnosis sits alarmingly between 2 to 5 years. Although there are existing treatments like pirfenidone and nintedanib, their efficacy is limited and they often result in gastrointestinal side effects, including nausea and diarrhea. These challenges lead to poor long-term adherence among patients, showcasing a dire need for more effective and better-tolerated therapeutic options.
The Promising Features of AK3280
AK3280 is described as an optimized small-molecule anti-fibrotic agent, which has shown promising results in earlier trials conducted in China. In a Phase 2 proof-of-concept study, AK3280 not only demonstrated a statistically significant improvement in forced vital capacity (FVC) at Week 24 but also showed enhancements in various lung function metrics. Importantly, one of the most notable findings was its favorable safety profile, which did not show the gastrointestinal side effects commonly linked to existing therapies.
The approval to move forward with a Phase 2 study in the U.S. represents a pivotal moment for ArkBio and its aspirations to provide new hope for patients with IPF. The upcoming multi-center, randomized, partially double-blinded trial will assess the efficacy, safety, and pharmacokinetics of AK3280, ensuring comprehensive data collection to support potentially groundbreaking advancements in treatment options.
A Commitment to Innovation
ArkBio, founded in 2014, has been at the forefront of biopharmaceutical innovation, focusing on therapeutics for respiratory and pediatric conditions. Their commitment to advancing innovative treatments is reflected in their strategic collaboration with multiple multinational pharmaceutical partners and academic institutions, which fortifies their research and development pipeline.
In addition to AK3280, ArkBio boasts a range of other promising assets, such as Ziresovir, which is recognized as the first direct-acting antiviral for RSV that has yielded positive Phase 3 results.
Looking Ahead
With the FDA’s blessing, ArkBio is set to take a monumental step in the quest to improve life quality for those battling idiopathic pulmonary fibrosis. The Phase 2 clinical trial of AK3280 is expected to yield crucial data, vital for future regulatory approvals and potential market introduction. As the trial progresses, there is cautious optimism that AK3280 might emerge as a therapeutic game-changer, providing effective management for patients afflicted by a disorder that has long been challenging to tackle.
For more information about ArkBio and their ongoing research, visit www.arkbiosciences.com.
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.