Phenylketonuria Market Set to Thrive by 2034 with New Therapies and Increased Awareness
The Future of the Phenylketonuria Market
The landscape of the phenylketonuria (PKU) treatment market is on the verge of a revolutionary transformation, projected to evolve significantly by the year 2034. This positive trajectory is largely attributed to several innovative therapies currently under development and rising rates of diagnosis worldwide.
Overview of Phenylketonuria
Phenylketonuria is a rare genetic disorder characterized by the inability to metabolize an amino acid known as phenylalanine. If left untreated, high levels of phenylalanine can lead to severe neurological issues. The importance of early diagnosis is underscored by newborn screening programs, which have improved detection rates globally, leading to a marked increase in the number of diagnosed cases.
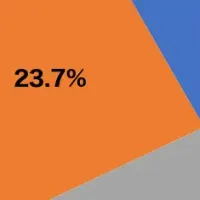
Recent reports suggest that the total market size for PKU treatment is expected to grow positively, particularly in leading markets such as the United States, EU4 (Germany, Italy, France, and Spain), the United Kingdom, and Japan. The U.S. alone is predicted to include approximately 18,800 diagnosed PKU cases in 2024, highlighting the immense potential for future market expansion.
Drivers of Market Growth
Several factors are propelling the growth of the PKU market:
- - Increased Awareness and Diagnosis: There is a growing global emphasis on newborn screening, which fosters early detection and care pathways for untreated PKU.
- - Emergence of Novel Therapeutics: Many promising treatments are being developed, including NGGT002 from NGGT, JNT-517 from Jnana Therapeutics, and Pegvaliase from BioMarin. Abundant research is currently aimed at generating alternative therapies, including gene therapy and large neutral amino acid supplementation.
Treatment Modalities
Currently, PKU treatment comprises both non-pharmacological and pharmacological approaches designed to manage blood phenylalanine levels effectively. Notably, the two FDA-approved therapies—PALYNZIQ (pegvaliase) and Sephience—use distinct mechanisms to reduce phenylalanine levels.
- - PALYNZIQ: This enzyme substitution therapy employs phenylalanine ammonia-lyase (PAL) to metabolize phenylalanine into non-toxic substances.
- - Sephience: This product acts as a precursor for the enzymatic cofactor BH4, essential for the proper functioning of the phenylalanine hydroxylase enzyme.
Additionally, JAVYGTOR, a generic form of sapropterin dihydrochloride, gained FDA approval in September 2022, enhancing access to BH4-based treatment options. The development of new entrants into the market is set to create further opportunities to diversify treatment options as traditional therapies face limitations.
Competitive Landscape
Among the numerous therapies under clinical trials, NGGT002 is an investigational gene therapy currently being assessed in Phase I/II. Early results are promising, with several patients maintaining target phenylalanine levels for an extended duration after treatment.
Jnana Therapeutics' JNT-517 also holds promise as a first-in-class oral treatment designed for a broad range of PKU patients. It uniquely targets the SLC6A19 transporter to help decrease blood phenylalanine levels effectively.
As new modalities enter clinical practice, the market's structure will undoubtedly shift, suggesting a growing imperative for medical innovation.
Recent Advancements
Significant developments have already commenced in this evolving market:
- - December 2025 saw the Japanese Health Ministry approve Sephience for PKU treatment across all age groups.
- - In October 2025, BioMarin sought expanded FDA approval for pegvaliase to include adolescents.
- - Data from the Phase III APHENITY trial reaffirms the efficacy of sepiapterin in enhancing dietary intake of phenylalanine.
These advancements outline a promising future for the PKU market, driven by increased innovation, improved diagnostics, and evolving therapeutic options.
Conclusion
In conclusion, the burgeoning phenylketonuria market reflects a confluence of increased awareness, innovative treatment options, and ongoing research. Stakeholders in the healthcare and pharmaceutical sectors are well-advised to monitor developments in PKU as they promise to redefine management approaches and ultimately enhance patient outcomes. As scientific inquiry continues to advance, new standards of care for PKU are on the horizon, poised to open the door to a more hopeful future for those impacted by this metabolic disorder.
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.