
Breakthrough in Mid-Temperature Superionic Conductors Enhances Fuel Cell Efficiency
Introduction
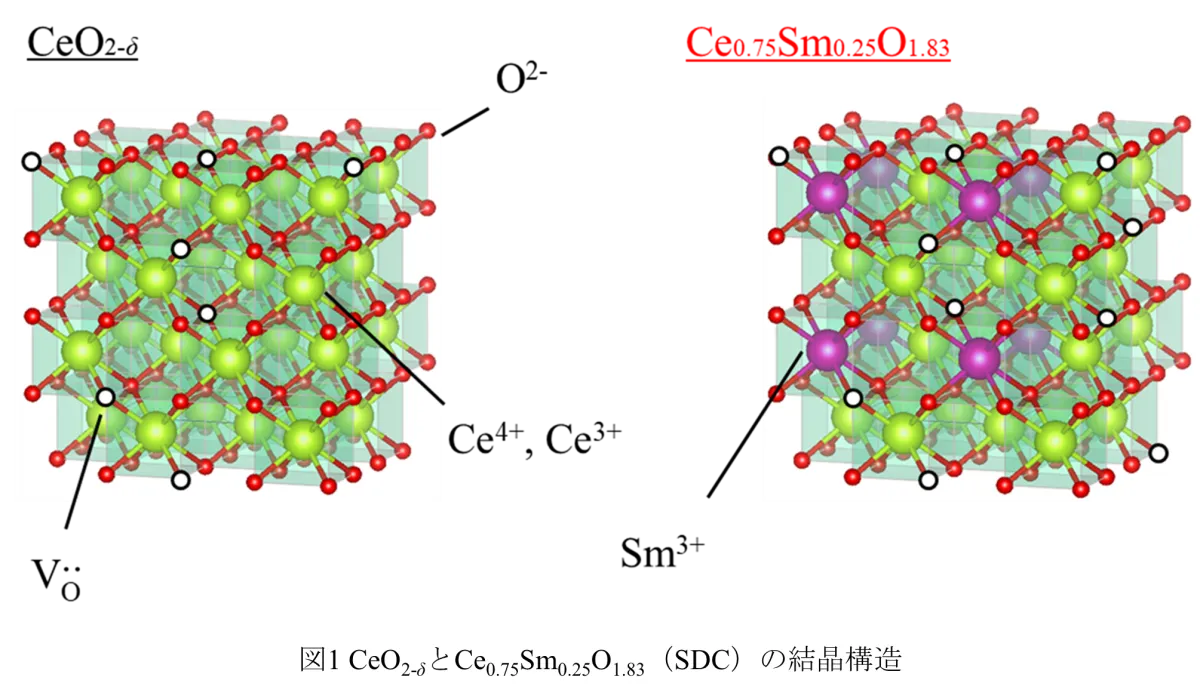
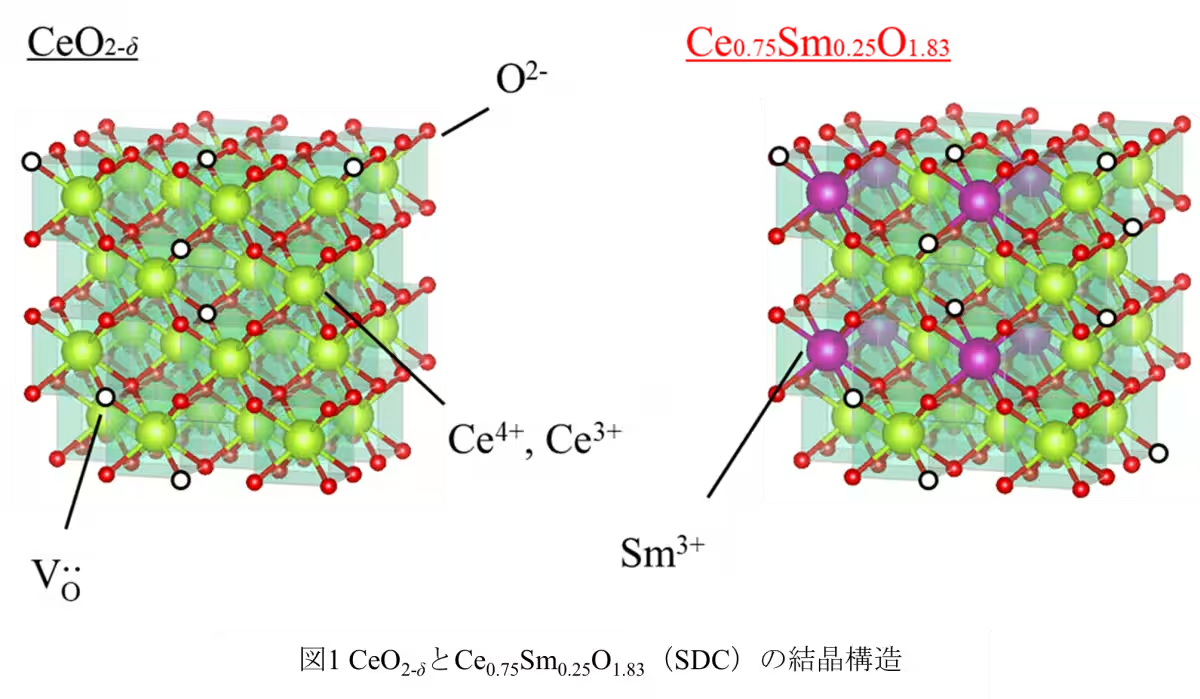
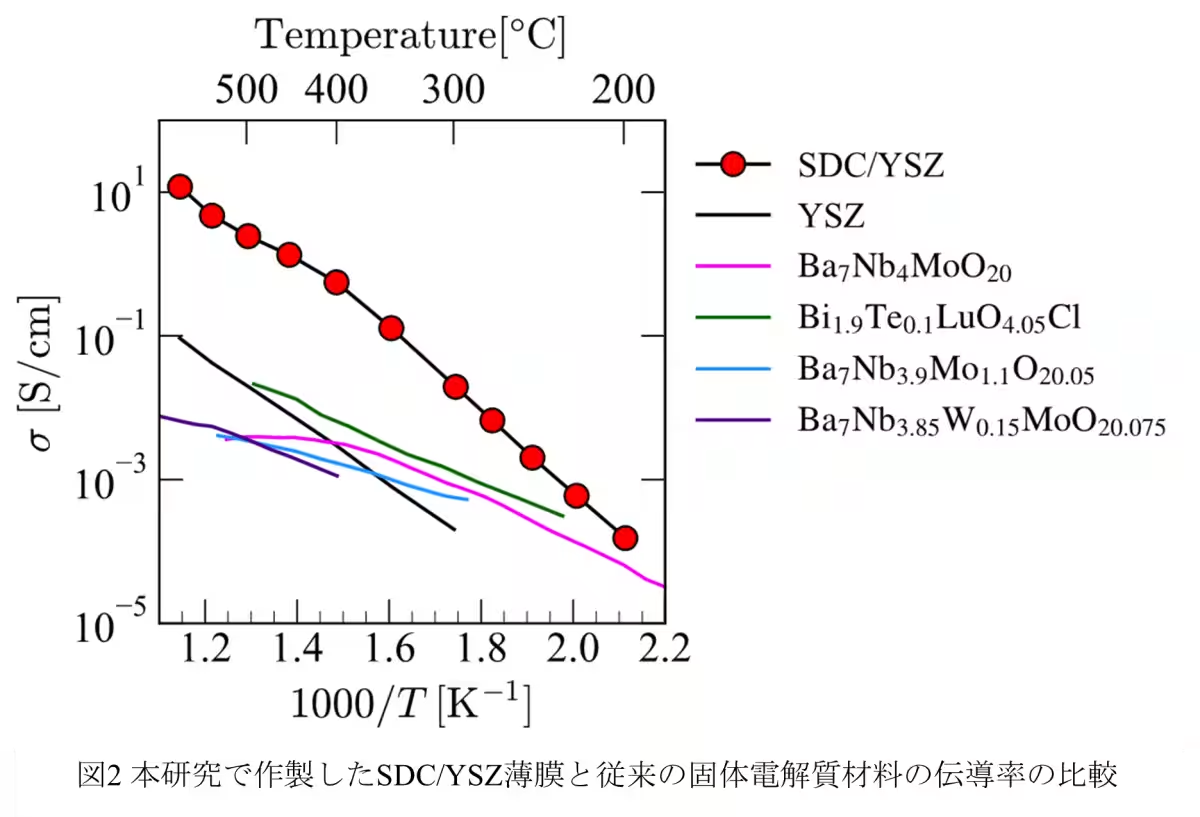
Recent developments in fuel cell technology have highlighted the need for efficient superionic conductors that operate in mid-temperature ranges. A team of researchers from Tokyo University of Science, led by Professor Tohru Higuchi, has unveiled a remarkable breakthrough in this field. They have successfully developed an a-axis-oriented Sm3+-doped CeO2 (Ce0.75Sm0.25O2-δ: SDC) thin film that exhibits extraordinary oxide ion conductivity exceeding 10-2 S/cm between the critical temperature range of 200 to 550℃. This breakthrough promises substantial advancements in the performance and application of solid oxide fuel cells (SOFCs).
Research Overview
The collaborative research group, which includes Ryota Morizane and Riku Tabuchi from Tokyo University of Science, alongside Daisuke Shiga from Tohoku University, focused on synthesizing SDC thin films on (100) oriented Yttria-stabilized Zirconia (YSZ) substrates. Using RF magnetron sputtering techniques, they produced thin films approximately 20 nm thick and meticulously evaluated their ionic conductivity and oxygen vacancy characteristics. The results revealed a remarkably low bulk resistance of about 0.05 kΩ cm at 300℃, indicating outstanding ionic transport capabilities. The films demonstrated high ionic conductivity exceeding 10-2 S/cm and achieved an ionic transport number of 0.96, confirming their status as purely oxide ion conductors with minimal electronic conduction.
Mechanism of Ionic Conductivity
The impressive conductivity exhibited by the SDC/YSZ thin films is attributed to three primary factors:
1. A significant number of oxygen vacancies (δ = 0.17) formed on the b-c plane facilitates effective ion transport.
2. The energy gap of approximately 2.6 eV hinders electron conduction.
3. Strong Coulomb repulsion among Ce 4f electrons ensures a pure ionic conduction environment. This combination of factors illustrates the potential for the SDC/YSZ system to serve as a practical electrolyte material in SOFCs and all-solid-state electrochemical transistors.
Potential Impact
The demand for mid-temperature working SOFCs is rising due to the significant advantages they hold in terms of efficiency and operational costs. Traditional SOFCs function at high temperatures, typically above 700℃, necessitating the continuous development of new electrolyte materials that can perform optimally at lower temperatures. The SDC thin film represents a significant advancement toward achieving this goal, potentially resulting in more cost-effective and efficient SOFC systems.
The significance of this research was underscored when the findings were published online on December 19, 2025, in the Journal of the Physical Society of Japan. Professor Higuchi expressed optimism regarding the application of the newly developed electrolyte materials, indicating their potential role in enhancing power generation capabilities across various solid-state systems.
Background of Solid Oxide Fuel Cells
SOFCs operate through the electrochemical reaction of hydrogen and oxygen, generating power by enabling oxide ions to move through a solid electrolyte between the anode and cathode. The high efficiency of this system, combined with its ability to utilize unreacted gases and waste heat, facilitates its implementation in cogeneration systems. The current leading materials, such as YSZ and CeO2, have demonstrated high stability and oxide ion conductivity; however, the need for more cost-effective alternatives remains pressing. The research team addressed this challenge by innovating a new oxide electrolyte that operates efficiently within a mid-temperature spectrum.
Future Directions
The high-performance characteristics of the SDC/YSZ thin films open new pathways for the development of advanced solid oxide systems. According to the research team, continued work on these materials can lead to next-generation SOFCs that operate efficiently in the mid-temperature range and also explore applications in all-solid-state electrochemical transistors. Research efforts will continue to focus on optimizing these materials and potentially improving their performance under varying operational conditions.
Conclusion
This pioneering study not only provides a valuable contribution to the fields of materials science and fuel cell technology but also reflects the increasing collaboration between leading universities and research institutions. The ability to innovate in materials like SDC thin films positions the scientific community favorably for sustainable energy solutions, ultimately paving the way for a future dominated by cleaner and more efficient power generation technologies.
Topics Energy)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.