
Kenai Therapeutics Begins Phase 1 Clinical Trial for RNDP-001 in Parkinson's Disease
Kenai Therapeutics Announces Phase 1 Trial for Parkinson’s Disease
Kenai Therapeutics, a pioneering biotech firm focused on innovative treatments for neurological disorders, has officially begun the Phase 1 clinical trial for its groundbreaking neuron replacement therapy, RNDP-001. This milestone is significant as it represents a potential shift in how Parkinson’s disease is treated, moving away from merely managing symptoms to actually addressing the underlying neuronal loss that characterizes the condition.
Trial Introduction
On December 15, 2025, Kenai Therapeutics announced that the first patient has been dosed in the REPLACE™ trial, which targets moderate to moderate-severe idiopathic Parkinson's disease. This advancement follows the approval of Kenai's Investigational New Drug (IND) application by the U.S. Food and Drug Administration (FDA), allowing the clinical evaluation of RNDP-001. This therapy aims to restore movement capabilities by replacing the dopamine-producing neurons lost due to the progression of the disease.
The urgency behind this project is underscored by the FDA granting Fast Track designation, indicating the recognized need for innovative treatment solutions in the field of Parkinson’s disease. Fast Track status enables faster development and review processes, which can significantly benefit patient care timelines.
Therapeutic Focus
Kenai's approach is especially noteworthy because current treatments primarily focus on alleviating symptoms rather than repairing the neurological damage caused by the disease. The CEO, Nick Manusos, stated, "RNDP-001 aims to replace lost neurons, repair damaged neural circuits, and ultimately restore motor function, offering hope for a better quality of life for patients suffering from the disease."
Trial Details
The REPLACE™ clinical trial is designed as an open-label, multi-center study that evaluates not only the safety and tolerability of RNDP-001 but also seeks to gather brain imaging evidence of cell survival and engraftment following the therapy. The trial will enroll up to 12 adult patients who are diagnosed with moderate to moderate-severe idiopathic Parkinson's disease. Early safety and efficacy data from the trial are anticipated to emerge in 2026, reflecting the therapy’s potential impact on this long-standing and debilitating condition.
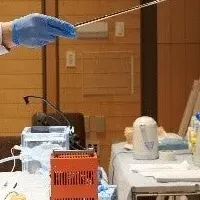
The innovative administration technique involves a precision neurosurgical procedure, ensuring targeted delivery of the neuron replacement cells to specific areas of the brain, where they can replace the damaged dopaminergic cells crucial for movement control.
Significance of RNDP-001
Dr. Howard Federoff, Chief Medical Officer of Kenai Therapeutics, emphasized the importance of this trial, remarking that the research behind RNDP-001 is profound and suggests that replacing dopaminergic neurons could significantly change treatment methods for Parkinson’s disease. This new direction in therapy could help address the fundamental issues of neuronal loss that define the disease's progression.
Collaboration and Innovation
Kenai’s innovation is further supported by its exclusive partnership with FUJIFILM Cellular Dynamics, Inc., which specializes in developing high-quality human induced pluripotent stem cells (iPSC). This collaboration allows Kenai to produce standardized and scalable cell therapies that can meet growing global demands, ensuring consistency and reliability for clinical applications.
Regulatory Support
The FDA’s Fast Track program aids in accelerating the path for therapies aimed at serious conditions like Parkinson’s disease, allowing for closer and more frequent interactions with regulatory authorities. This expedited process provides a pathway for Kenai Therapeutics to bring RNDP-001 to market as swiftly and efficiently as possible, potentially creating new hope for patients with Parkinson’s disease facing limited treatment options.
Conclusion
The initiation of the RNDP-001 trial marks an important leap forward in Kenai's mission to redefine how Parkinson’s disease is treated and illustrates their commitment to addressing patient needs at the fundamental level. As research and development continue, the hope remains that RNDP-001 will pave the way for transformative changes in the treatment and management of this complex and challenging condition.
Topics Health)








【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.