
Rybrevant® Gains Approval for NSCLC Treatment with Chemotherapy for EGFR Mutations
Johnson & Johnson's Rybrevant® Receives Approval for Chemotherapy Combination Therapy
On April 19, Johnson & Johnson (or Janssen Pharmaceutical K.K.) announced that Rybrevant® (amivantamab) has been approved for use in combination with chemotherapy for patients with unresectable and relapsed non-small cell lung cancer (NSCLC) that is positive for EGFR mutations. This new regimen is targeted at patients whose condition has worsened following treatment with EGFR-TKI alone, providing a significant new treatment option.
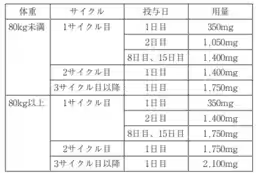
The recent approval allows Rybrevant® to be combined with chemotherapy agents carboplatin and pemetrexed for greater effectiveness. In clinical trials, this combination showed notable efficacy, reducing the risk of disease progression or death by 52% when compared to chemotherapy alone. The combination therapy resulted in a median progression-free survival (PFS) of 6.3 months versus 4.2 months for the chemotherapy group.
EGFR mutations are a common driver mutation found in approximately 80-85% of lung cancer cases overall, with EGFR-TKI monotherapy being a standard treatment. However, less than 20% of patients treated with TKI monotherapy for advanced NSCLC go on to survive five years past the initial diagnosis, predominantly due to acquired resistance. As a result, there has remained a significant unmet need for effective treatment options for patients progressing post-TKI treatment.
The approval of Rybrevant® is based on findings from the international, pivotal Phase III MARIPOSA-2 study, which evaluated its effectiveness and safety against other therapies in patients with specific EGFR mutations following TKI treatment failure. In this study, patients treated with the Rybrevant® plus chemotherapy regimen exhibited a higher overall response rate (ORR) of 53% compared to 29% in the chemotherapy-only group.
Rybrevant® is positioned as the only Category 1 treatment option recommended by the National Comprehensive Cancer Network® (NCCN®) for patients with EGFR mutations in NSCLC who experience disease progression post-TKI treatment. Furthermore, the American Society of Clinical Oncology's treatment guidelines strongly recommend this combination therapy as a second-line option. It is noteworthy that the safety profile associated with Rybrevant® when used with chemotherapy aligns consistently with that of the drugs used separately, highlighting its clinical safety.
Shuhei Sekiguchi, President of Janssen Pharmaceutical K.K., expressed optimism regarding the approval, stating, "Following the introduction of Rybrevant® last year, Johnson & Johnson continues to innovate and address unmet needs in the treatment of non-small cell lung cancer. With this latest approval, Rybrevant® provides a valuable new treatment alternative for patients with advanced disease who have already undergone TKI therapy. We hope this therapy will lead to advancements in lung cancer treatment in Japan, providing new hope to many patients."
Dr. Toshiaki Takahashi of the Shizuoka Cancer Center also emphasized the clinical benefits this approval heralds for patients, stating, "The approval presents an important new option for patients with NSCLC who have experienced disease progression following TKI therapy."
This comprehensive push to offer advanced therapies represents a positive stride towards improving treatment outcomes for lung cancer patients. With Rybrevant® now positioned to offer a dual approach alongside chemotherapy, there is renewed hope for patients facing the challenges of EGFR mutation-positive NSCLC. As the battle against lung cancer continues, innovations like Rybrevant® promise to play a critical role in enhancing patient quality of life and outcomes in the coming years.
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.