
New Findings on Hydrate Structures Reveal Flexibility in Water Molecule Frameworks
Introduction
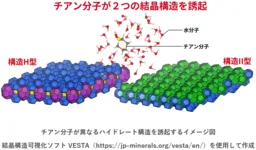
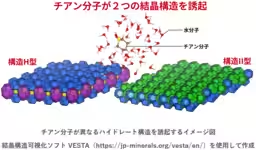
In a groundbreaking study, researchers from the National Institute of Advanced Industrial Science and Technology (AIST) have uncovered a remarkable phenomenon regarding hydrate structures. Specifically, it was found that two distinct types of hydrate crystalline structures can form from the same water molecule type when combined with thiophene (C5H10S). This discovery offers new insight into the design of environmentally friendly energy and material applications.
Key Discoveries
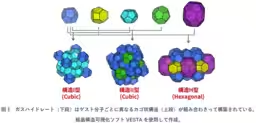
- - It was revealed for the first time that when the water molecule incorporates thiophene, it can generate two different hydrate crystalline structures: Structure II and Structure H.
- - This finding suggests that even a single type of molecule can lead to multiple crystalline structures. This directly challenges the previous assumption that hydrate structures are strictly determined by the size and shape of guest molecules.
- - The implications of this study indicate a promising future for utilizing water molecules in new functional material designs for CO2 storage and separation technologies.
The Significance of Hydrates
Hydrates are crystalline structures formed when water molecules encapsulate guest molecules like methane or CO2. They are characterized by their unique properties, such as being capable of gas storage and separation. The versatility of these crystalline forms makes them potential candidates for solving energy and environmental challenges. This study marks a significant step forward in our understanding of these structures.
Research Background
The research, led by Dr. Yusuke Shin from AIST and Professor Makoto Kida from Kitami Institute of Technology, emphasizes that water is a crucial resource not only for sustaining life but also for industrial activities. Hydrates have been traditionally thought to form specific crystalline structures based on the sizes and shapes of guest molecules. For instance, it has been understood that only certain configurations are possible for different guest molecules.
Breakthrough Findings
This research has shown that thiophene, while not forming hydrates by itself, can exist alongside smaller guest molecules like methane to create a unique crystalline structure. This flexibility challenges the long-held belief that each guest molecule correlates to a predetermined hydrate structure.
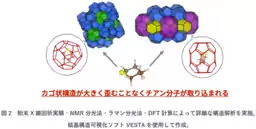
Utilizing advanced techniques such as powder X-ray diffraction, NMR spectroscopy, Raman spectroscopy, and density functional theory (DFT) calculations, the researchers were able to analyze the arrangement of the thiophene molecules within the hydrate cages. They discovered that thiophene consistently assumed a chair conformation across the different hydrate structures, indicating that it adapts naturally without excessive distortion of the cage structures.
Implications for Future Research
The findings present a notable advancement in understanding the hydrogen bonding network of water molecules and suggest a greater flexibility than previously acknowledged. The next steps for this research will involve exploring the molecular level origins of the two different structures and further elucidating the mechanisms behind hydrate formation and hydrogen bonding diversity.
The innovative use of water molecules in this manner could potentially lead to efficient designs for materials that engage in CO2 storage and separation, thereby contributing to environmental sustainability.
Conclusion
In summary, this research not only deepens our understanding of hydrate structures but also opens the door to new functional material designs within the energy and environmental domains. Moving forward, these insights could be pivotal for developing technologies aimed at mitigating climate change through CO2 management.
For the complete research findings, refer to the publication in Small Structures dated October 17, 2025.
Topics Environment)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.