
LifeSignals' UbiqVue™ 2A System Gains FDA Approval for Enhanced Patient Monitoring
LifeSignals' UbiqVue™ 2A Multiparameter System: A Leap Forward in Patient Monitoring
LifeSignals, Inc. has reached a significant milestone in healthcare technology with the FDA Class II 510(k) clearance of its UbiqVue 2A Multiparameter System. This state-of-the-art system is designed to enhance patient monitoring through continuous and remote physiological data collection, ideally suited for both in-hospital and at-home environments.
The Need for Continuous Monitoring
In today's healthcare landscape, timely and precise monitoring of patients' vital signs plays a crucial role in effective treatment and patient safety. Traditional methods, which often rely on spot checks, can be time-consuming and potentially less accurate. The UbiqVue 2A Multiparameter System addresses these challenges by offering continuous, near real-time monitoring of critical health parameters.
How the UbiqVue 2A System Works
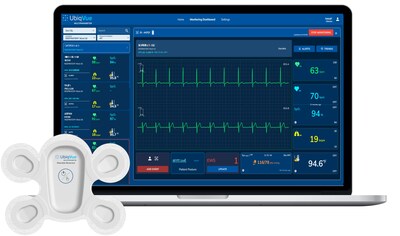
At the heart of the UbiqVue 2A System is a single-use, wearable biosensor that reliably monitors several key health metrics simultaneously. The device is equipped with a chest-based SpO2 sensor that measures oxygen saturation levels, alongside a comprehensive array of twelve monitored parameters including:
- - 2-channel ECG
- - Pulse rate
- - PPG (Photoplethysmography)
- - Respiration rate
- - Body temperature
- - Motion detection
This innovative technology allows healthcare professionals access to vital signs through a secure web portal, with alert notifications for any anomalies detected, thereby streamlining patient care.
Wirelessly Connected Data
The UbiqVue system enables healthcare providers to gather encrypted data from the biosensor, which is transmitted to a secure cloud-based system. This constant flow of information supports faster decision-making and enhances overall patient management strategies. With this technology, patient information can be monitored remotely, allowing for a more flexible and efficient approach to healthcare.
Testimonials from LifeSignals Leaders
Surendar Magar, CEO and Co-founder of LifeSignals, emphasized the significance of this FDA approval in transforming patient monitoring. He stated, "This [approval] marks a major milestone in LifeSignals' mission to deliver bedside patient monitor-like functionality through an affordable, single-use disposable biosensor, facilitating population health management."
Thomas Varghese, Chief Engineering Officer, echoed similar sentiments, highlighting the complexities tackled by the team in developing the UbiqVue System. He pointed out the importance of innovations in the realms of silicon design, signal processing, and wireless technology to meet the modern demands of healthcare.
In addition, Saravanan Balasubramanian, VP of Medical Technology Regulatory Operations, remarked on how this system combines the critical parameters of a bedside monitor with a low-cost, easy-to-use biosensor, fulfilling the need for accuracy and reliability in patient monitoring.
Future of Healthcare Monitoring
The introduction of the UbiqVue 2A System is expected to reshape individual patient care strategies and overall health management frameworks. LifeSignals aims to expand the capabilities of the UbiqVue system further, incorporating advanced artificial intelligence functionalities.
In collaboration with global partners, LifeSignals seeks to bring transformative health monitoring solutions to healthcare facilities and patients. With a commitment to innovation, the UbiqVue system not only promises to revolutionize how patient data is collected but also sets a new standard for the future of healthcare.
For more details, visit LifeSignals.

Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.