
Field Medical's FieldForce™ Ablation System Receives FDA Recognition for Ventricular Tachycardia Treatment
Transforming Cardiac Care
In a groundbreaking development, Field Medical, Inc. has announced that its innovative FieldForce™ Ablation System has received acceptance into the FDA's TAP Pilot program, alongside the prestigious designation of Breakthrough Device for the treatment of monomorphic ventricular tachycardia (VT) associated with scarring. This recognition is not just a commendation; it serves to illuminate the critical need for advanced solutions to combat potentially life-threatening vascular diseases affecting countless individuals globally.
Innovative Approach to Cardiac Ablation
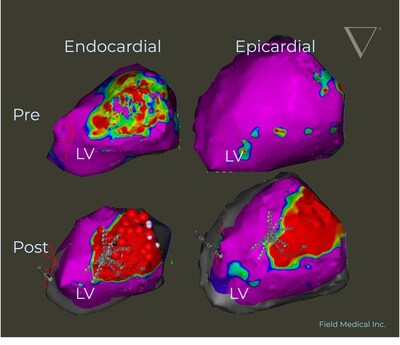
FieldForce™ stands out as the premier and sole pulsed field ablation (PFA) system specifically designed for addressing ventricular arrhythmias. The efficacy of catheter ablation has been consistently proven superior to pharmacotherapy in patients at risk of sudden cardiac death due to VT. Each year, sudden cardiac death accounts for approximately 450,000 fatalities in the United States alone, predominantly linked to VT conditions. This emphasizes the urgent medical need for more effective treatment methodologies.
Present-day pharmacological therapies often fall short, with studies indicating that 30% to 50% of patients with VT do not adequately respond to traditional treatment options. As such, the acceptance of Field Medical's device is particularly crucial, positioning it as a beacon of hope as medical science continues to seek innovative treatments.
Acknowledgment from Leaders in Electrophysiology
Dr. Steven Mickelsen, CEO of Field Medical, expressed profound optimism regarding these accolades, stating, "The FDA’s recognition of the TAP Pilot project and Breakthrough Device designation for the FieldForce™ Ablation System marks a significant milestone in our pathway to regulatory approval. It enables us to further our vision of equipping electrophysiologists with a next-generation focused PFA tool for rapid and accessible VT management."
Adding his voice, Dr. Vivek Reddy from the Mount Sinai Health System remarked on the importance of innovative technology in this field, emphasizing that the technology's recognition underscores the urgent demand for enhancements in treating complex and life-threatening tachycardias.
The FieldForce™ Impact
FieldForce™ utilizes a unique, single-point contact PFA catheter designed to deliver precise electrical fields, thus enabling targeted ablation in a quick and effective manner. The distinct FieldBending™ technology allows for both precise targeted lesions and deep transmural lesions in the ventricle.
This advancement is poised not only to redefine standard patient care but also to establish a new paradigm in treating VT. The commitment of Field Medical to innovation is evident as they extend their cutting-edge technology to more complex arrhythmias beyond merely atrial fibrillation.
Celebrating its inception in 2022, Field Medical has positioned itself at the forefront of the pulsed field ablation technology domain, demonstrating an unwavering dedication to enhancing patient outcomes. The company's commitment to drive advancements in cardiac care benefits millions worldwide affected by arterial and cardiac anomalies.
Looking Ahead
FieldForce™’s passage into the experimental status also highlights the need for continuous research and development in medical technologies that ensure patient safety and efficacy in treatment. As the journey progresses toward full regulatory approval, the excitement surrounding Field Medical's innovations continues to grow. The company aims to deliver remarkable outcomes for patients grappling with severe cardiac issues and to reshape the landscape of cardiac healthcare as we know it.
For further details, visit Field Medical's website or connect with them on LinkedIn and other platforms.
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.