

Innovative Use of Liquid Gallium for High-Efficiency Oxide Membranes in Oxygen Evolution Reactions
Innovative High-Efficiency Membrane Development
In a groundbreaking achievement, researchers in Japan have harnessed liquid gallium to craft high-entropy oxide (HEO) ultrathin membranes capable of enhancing oxygen evolution reactions (OER). This advancement presents a promising approach for future energy technologies, particularly in catalysts and battery applications.
Understanding High-Entropy Oxides
High-entropy oxides are distinguished by their uniform incorporation of five or more metallic elements, allowing for exceptional chemical stability, catalytic activity, and electrochemical properties that are difficult to achieve with single-material approaches. Commonly regarded as next-generation materials for catalysts and energy devices, the complexity of HEO structures has historically posed significant challenges in their synthesis.
Approach by Waseda and Nagoya Universities
The research team, led by Professor Yoshiyuki Sugahara from Waseda University and Professor Yusuke Yamauchi from Nagoya University, pioneered a novel synthesis method. By utilizing the oxide layer naturally formed on liquid gallium, they successfully fabricated HEO ultrathin membranes. The high affinity of the gallium oxide layer for various metal ions allowed for the efficient incorporation of essential components necessary for HEO formation.
During the transformation, gallium oxide is produced, which leads to introduced strain within the HEO membranes. This strain plays a critical role in lowering the free energy barrier for OER, thus enabling the development of highly efficient electrode catalysts. The resulting ultrathin membranes, with a large surface area and active sites, show considerable promise for elevating reaction efficiencies in OER-related applications.
Experimental Results
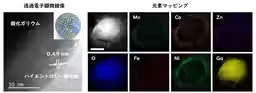
Utilizing transmission electron microscopy, the team examined the elemental mapping and composition of the fabricated HEO membranes. Their findings confirmed that the five metal elements were successfully concentrated on the outer area of the gallium oxide layer, maintaining a uniform dispersion crucial for stable membranes. Notably, the new synthesis technique resulted in an effective reduction of the membrane thickness to approximately 10 nm without compromising structural integrity.
The synthesized HEO membranes demonstrated remarkable catalytic activity for OER, surpassing conventional catalysts such as ruthenium oxide. Enhanced performance can be attributed to the unique characteristics of nanostructures and the beneficial effects of HEO and induced strain.
Implications and Future Directions
The implications of this research are far-reaching. The development of high-performance electrode catalysts can significantly improve the efficiency of the oxygen evolution reaction, a critical process in water electrolysis for hydrogen production. This methodology opens avenues for producing various compositions of HEO membranes, promoting their application in broader fields such as catalytic converters and energy storage systems.
As the study continues to develop, further explorations into the applications of HEO ultrathin membranes are anticipated, pushing boundaries beyond existing technologies. Researchers emphasize that the liquid gallium and its oxide layer can serve as a versatile platform for future innovations in material science and energy technology.
Conclusion
The study, published in Nature Communications, reaffirms the efficacy of using liquid gallium for synthesizing high-entropy oxide ultrathin membranes and illustrates their potential in renewable energy fields. As the global quest for sustainable energy solutions advances, innovations like these pave the way towards a more efficient future.
Further Reading
- - Zhang, W., Jin, H., Guo, Y., Cui, Y., Qin, J., Zhang, J., Yamauchi, Y., & Sugahara, Y. (2025). A universal approach for ultrathin high-entropy oxides regulated by Ga2O3 layers for oxygen evolution reaction. Nature Communications, DOI: 10.1038/s41467-025-60399-9.
This research was supported by the JST-ERATO project and the Special Research Fellow Program from the Japan Society for the Promotion of Science.


Topics Consumer Technology)









【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.