

New Service Launch: Speedy Solutions for Medical Device Regulatory Consultations
New Service Launch: The Regulatory Helpdesk
In a move to support the increasingly complex landscape of medical device regulations in Japan, the Tokyo Medical Device Agency has announced the launch of their new service, the "Regulatory Helpdesk". Set to commence on May 15, 2025, this initiative aims to address the rapid rise in demand for regulatory advice among medical device manufacturers and bolster the efficiency of their operations.
The implications of Japan’s Pharmaceutical and Medical Device Act (PMDA) compliance are significant. For manufacturers and producers of medical devices, understanding and adhering to these laws is paramount. This has become increasingly challenging due to a noticeable shortage of qualified personnel with the necessary expertise in regulatory matters, leading companies to rely more on outsourcing services.
Recognizing the current landscape, the Tokyo Medical Device Agency has positioned the Regulatory Helpdesk as a timely solution to address the needs of businesses lacking a readily available internal consultation team. Busy professionals often find themselves unable to seek clarification on pressing regulatory issues during their hectic schedules. This service is designed to streamline that process and facilitate better communication and support within the industry.
What the Regulatory Helpdesk Offers
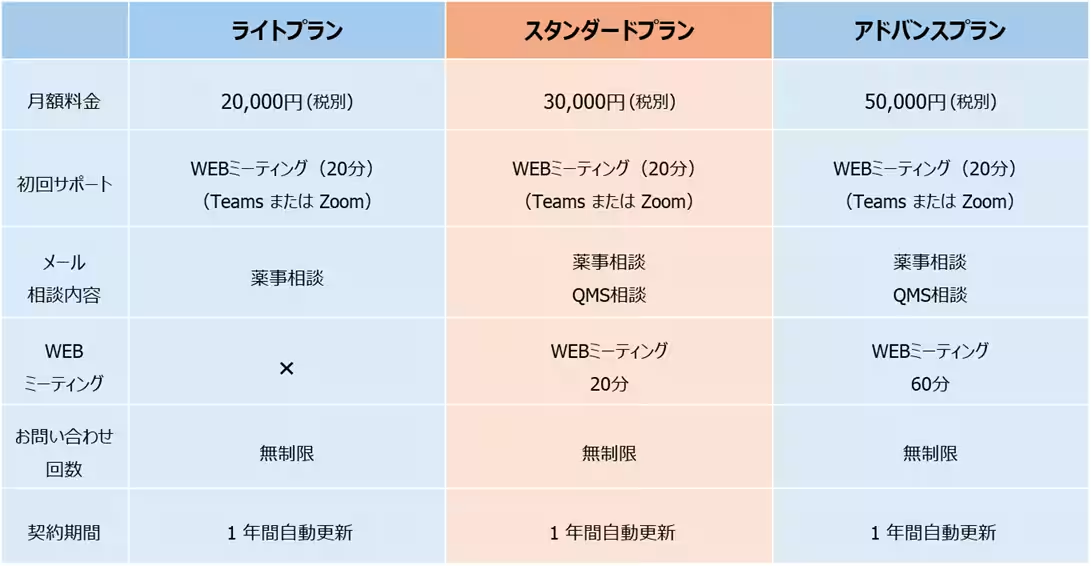
The Regulatory Helpdesk is tailored to meet diverse client needs, offering three distinct plans. By providing various options, this service assures a cost-effective approach that aims to eliminate unnecessary expenses for businesses seeking consultation.
One of the hallmarks of this service is its commitment to timely response. Through a dedicated email system, inquiries sent to the Regulatory Helpdesk will receive a first response within 24 hours (excluding weekends and holidays), ensuring that companies do not face lengthy delays. This rapid response time comes at a reasonable price, offering several advantages for the users:
1. Affordable options for medical device regulatory inquiries
2. Smooth resolution of uncertainties surrounding the PMDA and related regulations
3. Support in overcoming personnel shortages while enhancing operational efficacy
4. Assistance in improving the knowledge base of regulatory and quality assurance staff
The expertise of professionals answering queries is another key selling point of the Regulatory Helpdesk. All staff are well-versed in practical regulatory and quality assurance work, with experience in various certification organizations, ensuring that clients receive informed guidance tailored to their specific situations.
Additionally, the Regulatory Helpdesk provides an avenue for firms to elevate their staff's understanding of complex regulations through streamlined communication and support. This nurturing of knowledge is vital in fostering a workforce that can adeptly manage compliance directives and enhance overall organizational integrity.
Service Details and Pricing
More detailed information about the Regulatory Helpdesk, including an overview of services and pricing, can be found on the official website of the Tokyo Medical Device Agency at https://t-mda.com/helpdesk. The website also features an inquiry form for clients to smoothly navigate the contracting process. Pricing details are provided in a supplementary schedule. Notably, as part of the launch, a six-month trial contract option is available for businesses to explore the service firsthand.
The Regulatory Helpdesk aspires to complete 100 new contracts by the end of 2026, reflecting their commitment to facilitating compliance in the medical device sector. This proactive initiative positions the Tokyo Medical Device Agency as a leading support resource for manufacturers striving to navigate the complexities of regulatory requirements.
For further information, contact Tokyo Medical Device Agency, attention to CEO Maeda, at 042-707-6102 or via email at [email protected].

Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.