

SiteLi Cell Research Institute Collaborates with Saudi Arabia for Innovative Regenerative Medicine Trials
SiteLi Cell Research Institute's Groundbreaking Collaboration with Saudi Arabia
In a significant development in the world of regenerative medicine, SiteLi Cell Research Institute (Tokyo Stock Exchange: 3750), led by President Yoshihiro Hoshino, has announced a partnership with the esteemed King Abdullah International Medical Research Center (KAIMRC) in Saudi Arabia. This collaboration aims to initiate international clinical trials focusing on innovative ''one-day completion'' stem cell therapies, set to begin following the signing of a memorandum on June 26, 2025.
Revolutionizing Treatment with the One-Day Therapy
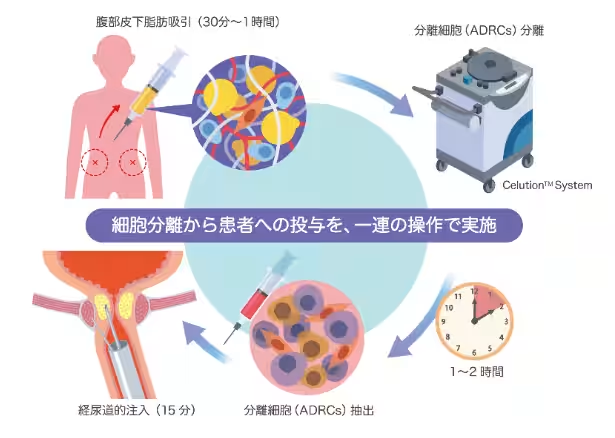
Traditionally, stem cell treatments required weeks or even months for cell cultivation after extraction from patients. Such lengthy processes not only presented significant costs but also added considerable waiting time, placing a heavy burden on patients. However, SiteLi's spin-off, ADRC Therapeutics, has developed an innovative 'Celation System' which allows for the extraction and preparation of adipose-derived regenerative cells (ADRCs) within just 90 minutes.
With this revolutionary ''one-day treatment,'' patients suffering from intractable diseases such as osteoarthritis, diabetic foot disease, Crohn's disease, and chronic pain can receive safe and effective treatment options derived from their own cells without prolonged waiting. This method notably reduces the risks of rejection and infection, as patients' own cells are utilized, and there is no need for cultivation.
A Historic Fusion of Saudi Vision 2030 and Japanese Technology
Saudi Arabia is currently implementing its Vision 2030 initiative, led by Crown Prince Mohammed bin Salman, which prioritizes the healthcare sector as a vital area for national development. KAIMRC is at the forefront of medical research efforts in the country, actively promoting the introduction of international medical technologies. This collaboration marks a pivotal milestone in the export of Japanese medical technologies.
The region is experiencing a surge in chronic diseases such as diabetes due to lifestyle changes, leading to an increased demand for innovative solutions. The introduction of Japan's stem cell technology is anticipated to address local healthcare challenges, reinforcing strategic partnerships between the two nations' healthcare sectors.
Establishing Sustainable Healthcare through Knowledge Transfer
The collaborative clinical trial will not only validate the safety and efficacy of ADRCs within local healthcare settings but also involve comprehensive educational programs for local medical professionals. A unique feature of the ADRC treatment is its independence from advanced culture facilities or specialized personnel, making it feasible to implement even in general medical institutions.
This accessibility will enhance medical reach in areas where specialized medical facilities are scarce, contributing significantly to improved healthcare access and reduced disparities. Japanese specialists will provide instruction on modern stem cell treatment techniques on-site, ensuring that healthcare providers in Saudi Arabia gain expertise in sustainable medical technology transfers. This initiative transcends mere technology provision, as it aims to contribute to the construction of regenerative medicine infrastructures within the Middle East.
Enhancing Competitiveness in the Global Market
The global stem cell treatment market is rapidly growing, with the Middle East emerging as a promising area for investment and economic expansion in healthcare. Should the clinical trial prove successful, ADRC Therapeutics is poised to establish a diverse business model in the Middle East, encompassing medical device sales, technology licensing, and physician training programs. Saudi Arabia will serve as a strategic starting point, with potential expansions into Qatar, Kuwait, and other GCC nations in the future.
Future Prospects
This memorandum signifies a long-term strategy for improving performance and corporate value for SiteLi Cell Research Institute. As Japan's innovative regenerative medicine technology gains international recognition, it will strengthen SiteLi's competitive edge in the global market, paving the way for further international integration.
About ADRC Technology
ADRC Therapeutics provides a state-of-the-art regenerative medicine technology that allows for the immediate administration of living cells extracted from a patient's own adipose tissue on the same day. The fully automated and closed-system procedures minimize infection risks while achieving quick results. The cells naturally migrate to damaged areas, promoting tissue regeneration and inflammation alleviation. Following the initial approval for treating male stress urinary incontinence, further developments for knee osteoarthritis and ischemic limb conditions are also underway.
Company Overview
ADRC Therapeutics, Inc.
Location: Chiyoda, Tokyo, 2-3-3 Koji-cho
Established: November 2002
Business: Development of regenerative medical technologies, manufacturing and sales of medical devices, and more.

Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.