
Recent Discovery of Novel β-1,2-Glucanase Families: Insights into Enzyme Evolution and Function
Recent Discovery of Novel β-1,2-Glucanase Families: Insights into Enzyme Evolution and Function
Introduction
Recent research conducted by a team from Tokyo University of Science has unveiled three previously unknown groups of β-1,2-glucanases, expanding our understanding of glycoside hydrolases (GHs). These findings not only shed light on the evolutionary aspects of these enzymes but also open avenues for novel applications in oligosaccharide synthesis.
Study Overview
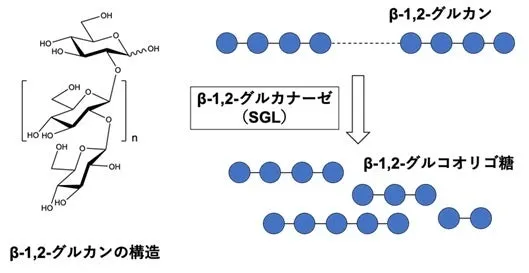
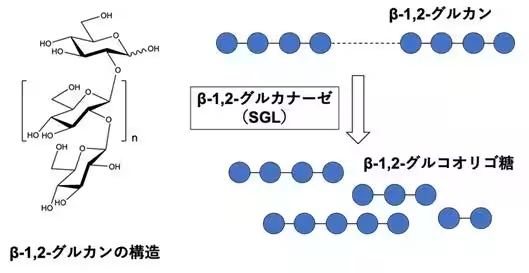
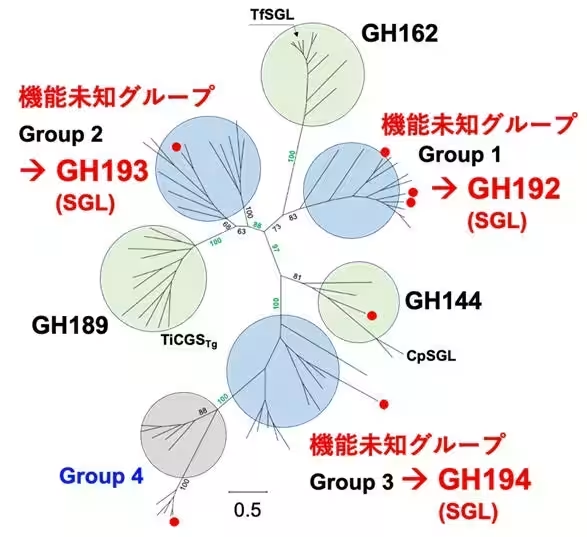
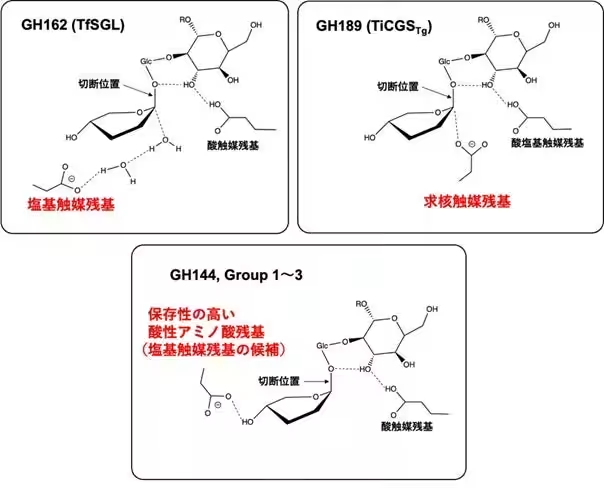
Conducted by Associate Professor Masahiro Nakajima and his research team, the study focused on the enzymatic breakdown of β-1,2-glucan, which produces β-1,2-gluco-oligosaccharides. Through extensive amino acid sequence homology searches, four novel groups (Group 1 to Group 4) were identified, with Groups 1 to 3 demonstrating activity against β-1,2-glucan.
The structural analysis revealed that enzymes from Groups 1 to 3 are distinct from known families GH144 and GH162, leading to the establishment of three new GH families (GH192, GH193, and GH194). This research highlights significant progress in exploring new enzymatic activities and understanding enzymatic reaction mechanisms.
Background
β-1,2-glucan is a natural glucose polymer produced by bacteria, crucial for various physiological functions such as symbiosis, osmoregulation, and immune evasion. Despite its significance, research on the enzymes involved in the synthesis and breakdown of β-1,2-glucan has been limited, particularly regarding their distribution and function in nature. Understanding how these enzymes operate at the molecular level can provide insights into their potential applications.
Methodology
The research team, which includes notable contributors ranging from experts in life sciences to artificial intelligence, employed a comprehensive approach. They utilized strains from soil bacterium Chitinophaga pinensis and the filamentous fungus Talaromyces funiculosus for their homology search. Their efforts led to the identification of four innovative groups of enzymes, specifically Groups 1 to 4, with the first three demonstrating the capability to hydrolyze β-1,2-glucan.
Functional analysis confirmed that the enzymatic activity of Groups 1 to 3 closely parallels known enzymes within the GH144 and GH162 families. Each group was characterized by distinct kinetic profiles and substrate specificities, underscoring their unique enzymatic functions.
Structural Insights
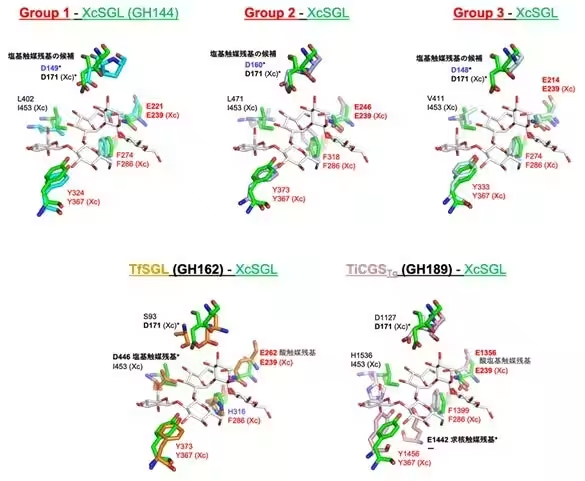
Advancing their investigations, the researchers conducted detailed structural analyses using advanced techniques, which revealed that the enzymes from Groups 1 to 3 possess a (α/α)6 barrel structure characteristic of known GH families. However, critical differences were noted in certain structural features, including the positioning and length of α-helices. This suggests a complex evolutionary trajectory where significant functional diversification has occurred within the SGL clan of enzymes.
Implications of Findings
The findings from this research carry substantial implications. By establishing new GH families, the study contributes significantly to the overall understanding of β-1,2-glucan metabolism. The evolutionary trajectory revealed through these studies emphasizes the intricate relationships and potential evolutionary innovations present within these enzymes. As enzymes can be pivotal in biotechnological applications, these discoveries hold promise for developing novel methods in oligosaccharide synthesis.
Future Directions
The ongoing exploration of glycoside hydrolases will likely catalyze further discoveries in this domain, leading to better-utilized enzymes in industrial applications. Associate Professor Nakajima remarked on the importance of these findings, stating, "Finding new enzyme groups not only contributes to academic knowledge but also paves the way for practical applications in biotechnology. Understanding the roles that these carbohydrates play is crucial, as they have various physiological functions."
As the research continues, the focus will remain on uncovering additional enzyme functions and their roles in metabolic pathways, enhancing our grasp of microbial interactions within ecological systems and their implications for agriculture and health.
Conclusion
The ongoing research underscores the evolving nature of enzymatic studies and the complexities of carbohydrate metabolism, serving as a pivotal step towards harnessing the potential of newly discovered enzymes for innovative applications.
- ---
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.