
LTT BioPharma and Our Company Join Forces for Drug Development
Collaborative Development Agreement with LTT BioPharma
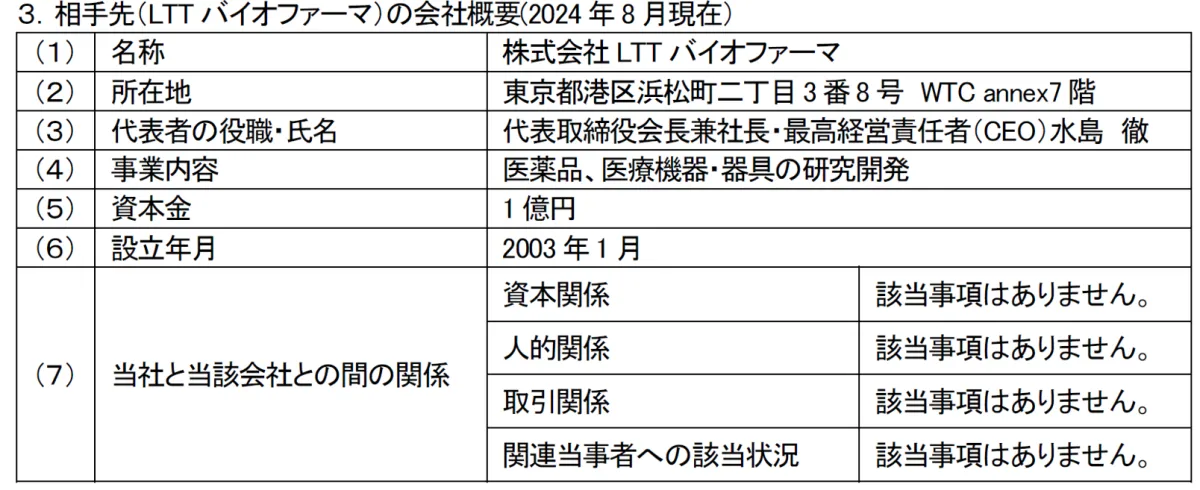
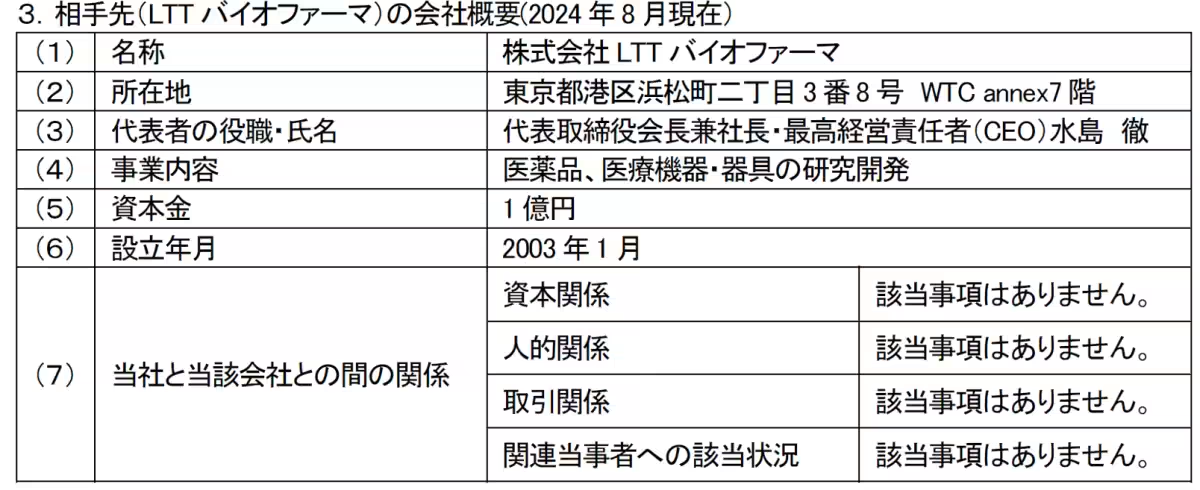
Our company is thrilled to announce the signing of a collaborative development agreement with LTT BioPharma, a venture stemming from St. Marianna University School of Medicine, based in Tokyo. This strategic move is aimed at reinforcing our mission to enhance the accessibility of innovative medications, particularly with the drug product known as PC-SOD, designed to combat peripheral neuropathy induced by chemotherapy.
Background and Goals
The signing of this agreement is a pivotal milestone in our Group's long-term business strategy, known as the "Alfresa Group Mid-Long Term Vision" which extends until fiscal 2032. This comprehensive strategy emphasizes strengthening and expanding our Total Supply Chain Service (TSCS) to create societal value through extending healthy lifespans, contributing to regional medicine, and fostering health care innovations.
LTT BioPharma, established in 2003 but with roots tracing back to the LTT Research Institute founded in 1988, specializes in smart drug development. They aim to deliver medications to clinical settings swiftly and cost-effectively by utilizing cutting-edge techniques such as Drug Repositioning (DR) to identify new therapeutic uses for existing drugs, combined with Drug Delivery Systems (DDS) for efficient drug administration.
A notable focus of LTT BioPharma is developing the bio-pharmaceutical PC-SOD, which targets Chemotherapy-Induced Peripheral Neuropathy (CIPN). This side effect presents a significant challenge for cancer patients undergoing chemotherapy, adversely affecting their quality of life and treatment persistence.
Development Continuation and TSCS Integration
By entering this collaborative agreement, our group commits to assist in developing, manufacturing, distributing, and selling PC-SOD through our cohesive TSCS framework. This collaborative effort aims to expedite the delivery of innovative medications to patients, enhancing drug access and its benefits to healthcare.
Key Details of the Agreement
Under this collaborative development contract, our company will share the financial burden of research and development while obtaining specific rights and operational priorities:
- - Pre-Market:
2. Priority rights for the distribution of clinical trial drugs.
3. Priority rights for Contract Research Organization (CRO) services.
- - Post-Market:
2. Priority rights for Post Marketing Surveillance (PMS) services.
3. Priority rights for packaging, labeling, and storage operations.
4. Distribution rights.
Looking Ahead
The collaborative effort is projected to have a minimal impact on our immediate financial outcomes, but we believe it will be significant in enhancing our corporate value in the medium and long term. We will continue to keep our stakeholders informed as new developments arise in relation to this collaboration.
This partnership is not merely a business agreement; it represents a collective ambition to innovate in the realm of pharmaceuticals, with the ultimate goal of improving patient outcomes in the fight against the challenging side effects of chemotherapy. We are excited about the journey ahead with LTT BioPharma.
For further updates regarding this agreement and its developments, please stay tuned.
Topics Health)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.