
Next-Generation Battery Materials Combining High Stability and Conductivity Through Ion Networks
Breakthrough in Battery Technology: Cooperative Ion Conduction in Random Substitutional Crystals
In the pursuit of advancing solid-state battery technology, researchers have made significant strides in developing solid electrolyte materials that balance safety and performance. The need for effective energy storage solutions is critical as we strive for a sustainable energy transition, and this recent study sheds light on a promising new material design principle.
The conventional approach to enhancing ion conductivity within solid electrolytes has required compromising structural stability. Typically, to boost ion conductivity, the crystal structure is relaxed, allowing ions to move freely. However, this often results in a detrimental decrease in the material's stability, posing a paradox that researchers have long grappled with.
A research team led by Professor Rei Kurita from the Graduate School of Science, Tokyo Metropolitan University, along with doctoral student Rikuya Ishikawa and Associate Professor Kyohei Takae from Tottori University, explored the behavior of lithium ions in random substitutional crystals. They employed molecular dynamics simulations to investigate how lithium ions move within a crystal framework that features randomly distributed atoms.
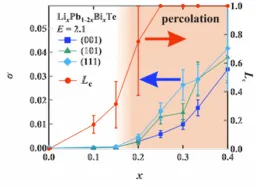
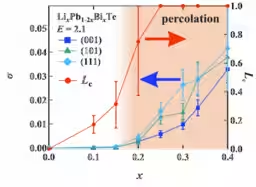
The findings revealed a critical threshold of lithium ion concentration—around 20%—at which the ions begin to form networks, drastically increasing conductivity. This threshold aligns with the percolation theory's predicted critical connectivity point, elucidating how ionic pathways spontaneously develop within the crystal structure.
Notably, the researchers demonstrated that this mechanism allows the material to achieve conductivity comparable to that of liquid electrolytes (6.8 × 10⁻³ S/cm) without destabilizing the crystal structure. Moreover, they confirmed isotropic ionic conduction, which does not depend on the crystallographic orientation, offering a new universal principle for the design of practical materials.
The implications of this study are far-reaching. It proposes a novel design guideline capable of harmonizing high stability and high conductivity—traits that have historically been considered mutually exclusive. The research suggests that the cooperative movement of ions within a network structure can create efficient pathways for ion flow without compromising structural integrity.
Study Background
As we aim to achieve a sustainable society, developing solid electrolyte materials that maintain safety while providing high performance is crucial. Current lithium-ion batteries utilize liquid electrolytes that offer good conductivity but present safety issues due to their flammable and reactive nature. This ongoing challenge has propelled research into all-solid batteries, which use solid materials for electrolytes, thereby enhancing safety and reducing risks such as leakage and thermal runaway.
Despite their promise, solid-state batteries have struggled with enabling rapid ion movement within solids. High conductivity and structural stability remain key hurdles in material design. Traditionally, to increase ionic concentration and conductivity, doping lithium ions was a common practice. However, excessive doping can destabilize the crystal structure, ultimately reducing conductivity.
The research team’s focus on random substitutional crystals introduces a new facet to this discussion. These materials feature a disorderly mix of multiple elements, and previous studies have reported enhanced mechanical strength and thermal resistance in related fields such as high-entropy alloys. By investigating how this disorder contributes to ionic conduction, the research aims to offer a solution to the stability-conductivity dilemma.
Research Methodology
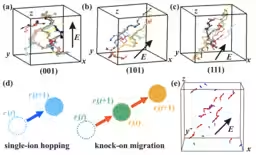
Using molecular dynamics (MD) simulations, the team explored ionic movement in LixPb₁−₂ₓBixTe, characterized by a NaCl-type structure. By varying lithium (Li⁺) concentration, they assessed the resulting ionic conductive behaviors. Given the small ionic radius of Li⁺, rapid movement within the crystal was anticipated.
The simulations confirmed that once lithium concentration exceeded 20%, conductivity surged dramatically. This threshold corresponds with the percolation theory’s predicted point of connectivity, indicating that a network of ions spans the entire crystal, facilitating continuous ionic conduction paths. In essence, when certain ion concentrations are reached, local movements begin to synchronize, resulting in a novel conduction mechanism characterized by “flowing through connectivity.”
Moreover, the stability of the crystal structure was maintained throughout the simulations, even when an electric field was applied. The direction of electric field application had minimal impact on the conductivity, indicating that the ionic network is uniformly distributed within the crystal.
Visualizing the individual ion movements also revealed a knock-on mechanism whereby adjacent Li⁺ ions move in a chain reaction, akin to a “domino effect.” This collaborative movement fosters efficient conductive pathways, enhancing overall performance. The resulting conductivity reached 6.8 × 10⁻³ S/cm, equivalent to that of liquid electrolytes, achieved without compromising thermal or chemical stability.
Significance and Future Impact
This groundbreaking research illustrates that a network structure of cooperating ions can spontaneously arise within disorderly crystal frameworks, facilitating concurrent high conductivity and high stability. The percolation theory’s underpinning of this phenomenon indicates a universal mechanism capable of achieving liquid-like conductivity without damaging the crystal structure, establishing a new material design paradigm.
This principle extends beyond lithium-based systems, offering exciting prospects for other ionic crystals and high-entropy materials, paving the way for advanced energy storage technologies and innovations in all-solid batteries.
Related Terminology
1. All-solid battery: A battery utilizing a solid electrolyte instead of a liquid one, providing enhanced safety.
2. Random substitutional crystal: A crystal structure wherein atoms are randomly replaced by different elements, potentially enhancing performance.
3. Percolation theory: A theoretical framework analyzing how interconnected elements form a cohesive structure in random arrangements.
Published Research
The findings were published in the Physical Review Materials by the American Physical Society on November 3rd, 2023. This research was supported by grants from the Japan Society for the Promotion of Science, underscoring its importance in the advancement of next-generation batteries.
Topics Consumer Technology)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.