

Launching Medical Software Consulting: A New Era for SaMD Startups and Investors
New Venture in Medical Software Consulting
Yoshio Sakai, a seasoned expert with nearly 40 years of experience in medical device software development and regulatory compliance, has announced the founding of Medical Software Consulting. Set to launch in June 2025, the firm is designed to offer comprehensive educational and consulting services specifically tailored for medical device and Software as a Medical Device (SaMD) developers as well as venture capitalists (VCs).
The aim of Medical Software Consulting is to assist startups in navigating the complexities of international regulations vital for medical device development, including compliance with IEC 62304, risk management (ISO 14971), and quality management (ISO 13485). The firm will also provide free explanatory videos on platforms like YouTube, focusing on critical topics in the field. These resources will be integrated with a paid educational management system intended to track participants’ progress, administer comprehension tests, issue completion certificates, and deliver educational reports.
The unique service is geared towards addressing pressing challenges faced by VC-backed startups, which often include issues such as compliance with Japanese pharmaceutical regulations, cybersecurity mandates, and the risks related to quality management. By facilitating early visibility and resolution of these compliance-related issues, Medical Software Consulting aims to maximize asset value at Exit through effective support of PMDA compliance and QMS development.
Services Offered
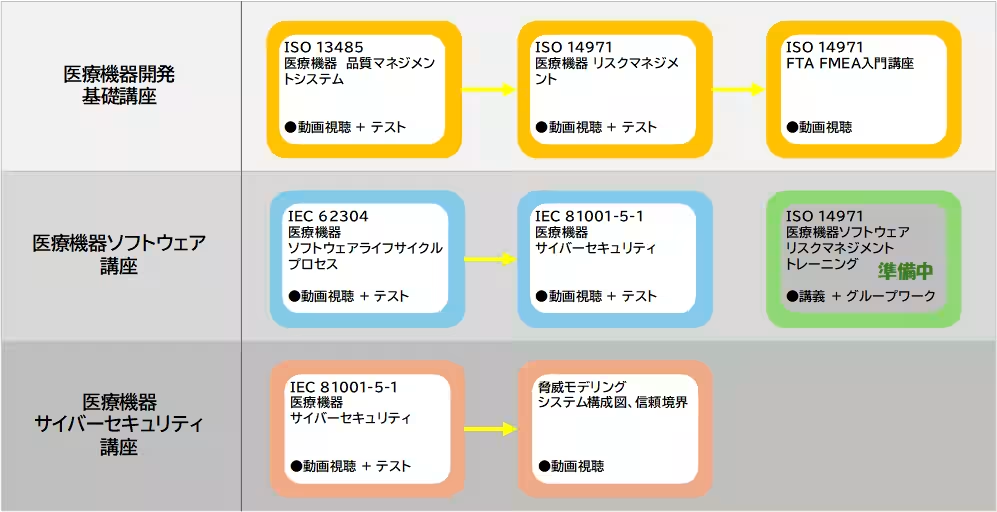
1. Educational Management Services
- Free Campaign Until September 30, 2025: Access to a variety of explanatory videos covering IEC 62304, IEC 81001-5-1, ISO 14971, ISO 13485, and introductory modules on FTA, FMEA, and threat modeling.
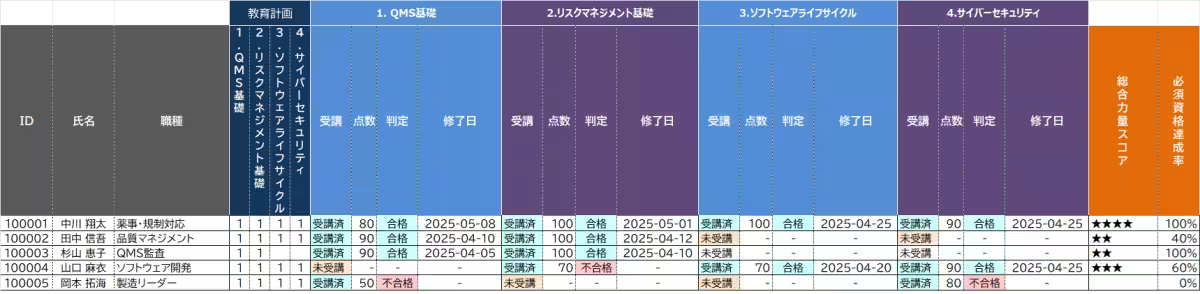
- User management via an educational management system (Gmail address required): Monitor viewing progress, conduct completion tests, issue completion certificates, and provide educational record reports.
- Pricing: Customized estimates available, currently under a free campaign until the end of September 2025.
Educational Course Flow
- Example of competency chart after system operation
- Application Form
2. Consulting Services
- Free Email Consultation (up to two consultations)
- Paid Individual Consultation: ¥20,000 per hour (excl. tax)
- Paid Advisory Contract: ¥500,000 per month (excl. tax, includes up to 50 hours)
- Support for QMS, IEC 62304, ISO 14971, and cybersecurity compliance, as well as PMDA consultation. Collaboration with partner firms for development and practical work is also available (additional fees may apply).
Founder Profile
Yoshio Sakai
- 39 years of experience in medical device software development (1986-2025)
- Responsible for software development in defibrillators, vital sign monitors, and DX systems at medical device manufacturers.
- Led corrective actions for products that received FDA Warning Letters, deepening knowledge of FDA regulatory compliance.
- Promoted IEC 62304 compliance, QMS, and cybersecurity standards within the internal tech support division since 2006.
- Active member and instructor in international standard committees (e.g., GHS, JIETA) under the Medical Device Manufacturers Association.
- Co-authored “IEC 62304 Practical Guide” (published by Jihou) in 2016 and wrote titles such as “How to Create Software Without Recalls” and “Mastering Embedded Software Engineering” (over 10,000 copies sold).
- Regularly shares insights on embedded software, regulatory standards, and education for software engineers through his blog, “Embedded Software Workshop.”
Message from the Founder
Sakai expresses his commitment: “To ensure that medical device startups are not obstructed by regulatory barriers on their journey to product commercialization, we provide support for regulatory compliance from the early stages of development. Our integrated approach combines educational content with consulting services, allowing companies to simultaneously foster talent and mitigate risks.”
Contact Information
Medical Software Consulting
Representative: Yoshio Sakai
Website: Medical Software Consulting
Email: [email protected]
Note: This service is intended for end-user companies of medical devices. Please refrain from utilizing it for consulting purposes.

Topics Other)










【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.