
Minaris Collaborates with Cell and Gene Therapy Catapult to Enhance Gene Therapy Delivery Technologies
Collaboration to Advance Gene Therapies
Introduction
In a significant step towards improving the effectiveness of cell and gene therapies, Minaris, a global leader in cell and gene therapy contract development and manufacturing organizations (CDMO), has announced a new partnership with the Cell and Gene Therapy Catapult (CGT Catapult). This collaboration aims to advance delivery methods for gene therapies, focusing on robustness, quality, and cost efficiency in manufacturing processes.
Details of the Collaboration
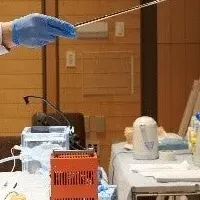
The partnership will see Minaris relocating its Center for Viral Vector Innovation into the CGT Catapult's collaborative laboratories located in London. This strategic move is expected to foster innovation and streamline the development of lentiviral (LV) assays and processes, which are crucial for gene therapy applications. The collaboration will leverage Minaris' established expertise in the LV space, enhancing CGT Catapult's existing capabilities, particularly in the adeno-associated virus (AAV) technology, another essential vehicle for delivering therapeutic materials into cells.
Benefits of Enhanced Delivery Technologies
The partnership is envisioned to yield increased analytical and process capabilities, thereby improving the productivity, safety, and overall performance of viral vector platforms. This is particularly important for the advanced therapy product developers and manufacturers who require robust support for LV and AAV platform technologies. The collaboration promises to create a more efficient and scalable method for producing transformative therapies, ultimately benefiting patients worldwide.
Matthew Durdy, the Chief Executive at CGT Catapult, expressed optimism about the collaboration, stating, "Minaris’ expertise in cell and gene therapies is a valuable contribution to the UK's advanced therapies industry. By working together to refine lentiviral development and manufacturing processes, we aim to ensure these methods are efficient and safe for producing life-altering therapies."
A Vision for the Future
Orla Cloak, CEO of Minaris, stated her excitement regarding the collaboration, emphasizing the alignment of both organizations' missions to accelerate treatments and expand patient access to innovative therapies. This partnership is expected to result in multiple initiatives that propel vector-based therapeutics toward commercial manufacturing.
About the Cell and Gene Therapy Catapult
The CGT Catapult is an independent technology and innovation organization focused on advancing the cell and gene therapy field. Their mission includes creating fruitful collaborations to tackle challenges and accelerate the development of advanced therapies that can change lives globally.
About Minaris
Minaris boasts over 25 years of experience in cell and gene therapy development, alongside over 40 years in biosafety testing. With a focus on excellence and reliability, Minaris operates across five global sites and collaborates closely with innovators to bring new therapies to market, ensuring high-quality production across various diseases.
Conclusion
This collaboration between Minaris and CGT Catapult stands as a pivotal moment in the gene therapy landscape. By harnessing their individual strengths, both organizations aim to pave the way forward for effective, accessible therapeutic solutions, ultimately enhancing the future of healthcare.
Topics Health)








【About Using Articles】
You can freely use the title and article content by linking to the page where the article is posted.
※ Images cannot be used.
【About Links】
Links are free to use.